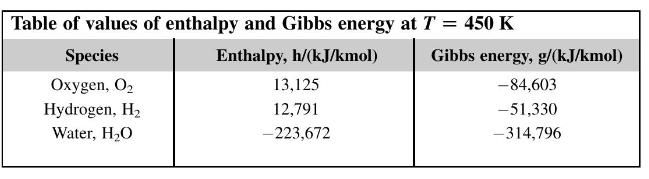
A hydrogen-oxygen fuel cell operates at a temperature of (450 mathrm{~K}) and the reactants and products are
Question:
A hydrogen-oxygen fuel cell operates at a temperature of \(450 \mathrm{~K}\) and the reactants and products are all at a pressure of 3 bar. Due to internal resistances the emf of the cell is only \(70 \%\) of the ideal value. Calculate the 'fuel consumption' of the ideal cell and the actual one in \(\mathrm{g} / \mathrm{kW}\) h.
An alternative method of producing electrical power from the hydrogen is to burn it in an internal combustion engine connected to an electrical generator. If the engine has a thermal efficiency of \(30 \%\) and the generator is \(85 \%\) efficient, calculate the fuel consumption in this case and compare it with that of the fuel cell. Explain why one is higher than the other.
[32.25 g/kW h; \(46 \mathrm{~g} / \mathrm{kW} \mathrm{h;} 116.2 \mathrm{~g} / \mathrm{kW} \mathrm{h}]\)
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





