(1.5 mathrm{kmol}) of feed one that is (40.0 mathrm{~mol} %) methanol and (60.0 mathrm{~mol} %) water and...
Question:
\(1.5 \mathrm{kmol}\) of feed one that is \(40.0 \mathrm{~mol} \%\) methanol and \(60.0 \mathrm{~mol} \%\) water and \(1.0 \mathrm{kmol}\) of feed two that is \(20.0 \mathrm{~mol} \%\) methanol and \(80.0 \mathrm{~mol} \%\) water will be processed in a simple batch distillation. The following three approaches have been proposed:
a. Mix the two feeds together, and do the batch distillation.
b. Do a batch distillation of feed one until the still pot concentration is \(20 \mathrm{~mol} \%\) methanol (same as feed two), add feed two, and then complete the batch distillation.
c. Do a batch distillation of feed one and a separate batch distillation of feed two. Add the two distillate products and the two still-pot products.
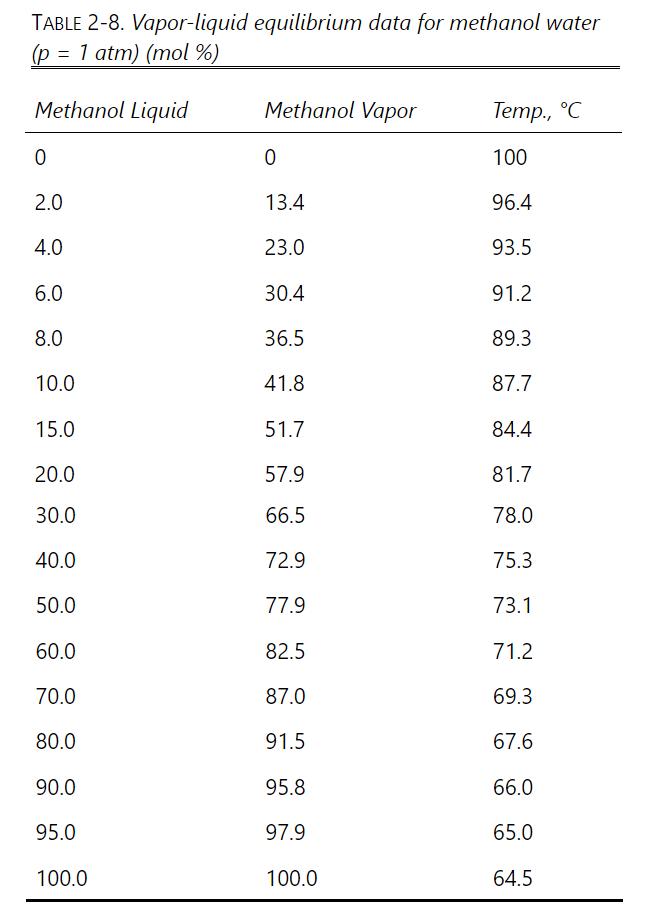
If we want \(\mathrm{x}_{\mathrm{W}, \text { fin }}=0.10\), do calculations for each method. Determine \(\mathrm{W}_{\text {final }}, \mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\), and compare the three methods. Equilibrium data are in Table 2-8. Logically, parts \(\mathrm{b}\) and \(\mathrm{c}\) should give the same result. If they do not, there is probably a numerical error from the use of Simpson's rule. Try dividing an area into two parts for more accuracy or use the Gaussian quadrature formula.
Table 2-8
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





