A simple batch distillation is used to process (1.0 mathrm{kmol}) of methanol-water feed into three distillate fractions
Question:
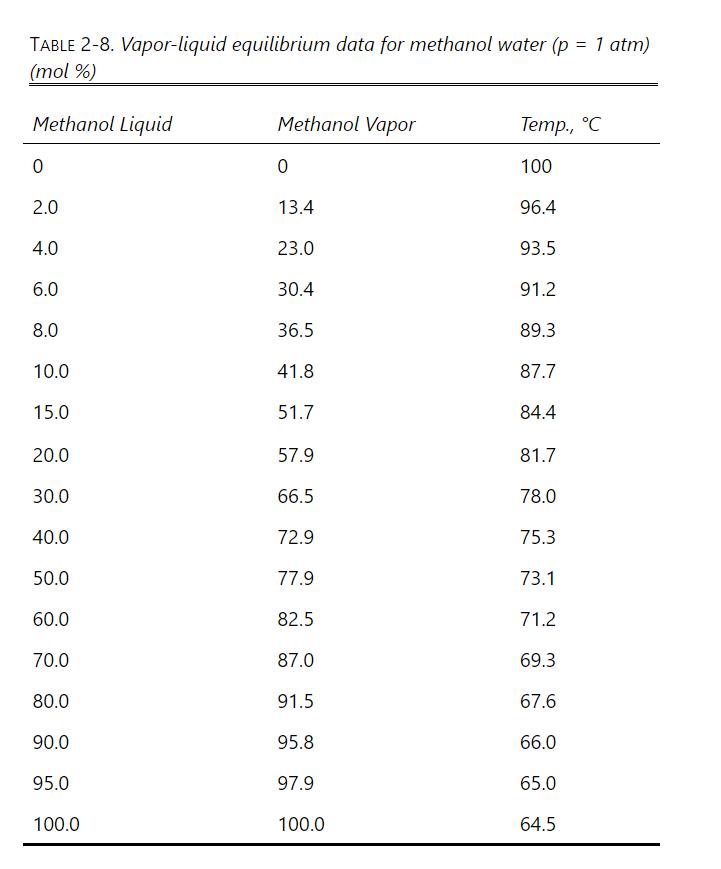
A simple batch distillation is used to process \(1.0 \mathrm{kmol}\) of methanol-water feed into three distillate fractions and a waste. The initial feed has \(\mathrm{x}_{F, \mathrm{M}}=\) 0.50 mole fraction. We want the average mole fractions of methanol in the distillate fractions to be \(\mathrm{x}_{\mathrm{D}, \text { avg, } 1}=0.70, \mathrm{x}_{\mathrm{D}, \text { avg, } 2}=0.60\), and \(\mathrm{x}_{\mathrm{D}, \text { avg, } 3}=0.40\). Equilibrium data are in Table 2-8.
Table 2-8
a. Find \(\mathrm{W}_{1, \text { fin }}, \mathrm{D}_{\mathrm{tot}, 1}, \mathrm{x}_{\mathrm{W}, \text { fin }, 1} ; \mathrm{W}_{2, \text { fin }}, \mathrm{D}_{\mathrm{tot}, 2}, \mathrm{x}_{\mathrm{Wfin}, 2} ;\) and \(\mathrm{W}_{3, \text { fin }}, \mathrm{D}_{\mathrm{tot}, 3}\), \(\mathrm{x}_{\mathrm{W}, \text { fin, } 3} \cdot\)
To make this problem tractable, the following procedure is suggested: Calculate relative volatility values \(\alpha_{M-\text { Water }}=\frac{y_{M} / x_{M}}{\left(1-y_{M}\right) /\left(1-x_{M}\right)}\) from equilibrium data at \(\mathrm{x}_{\mathrm{M}}=0.5,0.4,0.3,0.2,0.15\), and 0.10 . Over the different ranges of still pot mole fractions expected, calculate geometric average \(\alpha_{-}=\left(\alpha_{1} \alpha_{2} \ldots \alpha_{n}\right)^{1 / n}\) for each range.
Solve Eq. (9-13) on a spreadsheet to calculate \(\mathrm{W}_{\text {fin }}\) using the guessed value of \(\mathrm{x}_{\mathrm{W}, \text { final }}\). Calculate \(\mathrm{x}_{\mathrm{D}, \text { avg }}\) for each fraction. Use Goal Seek or Solver to find the value of \(\mathrm{x}_{\mathrm{W} \text {,fin }}\). If the average value of relative volatility used was for an obviously incorrect range, insert a corrected \(\alpha_{-}\), and repeat. Do this for each fraction.
Equation 9-13
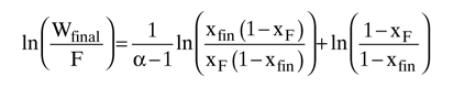
b. In the lab or plant, it is easier to collect fractions based on weight or time than based on mole fraction measurements. If heating is done at a rate of \(20 \mathrm{~kW}\), at what time in minutes should we switch from collecting the first fraction to collecting the second fraction? Required data are available in Appendix D in the back of book.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





