A simple batch still (one equilibrium stage) separates 100 moles of a 10.0 (mathrm{mol} %) methanol and
Question:
A simple batch still (one equilibrium stage) separates 100 moles of a 10.0 \(\mathrm{mol} \%\) methanol and \(90.0 \mathrm{~mol} \%\) water feed. The final bottoms concentration is \(1.0 \mathrm{~mol} \%\) methanol. VLE data are in Table 2-8. \(\mathrm{p}=1.0\) atm.
Table 2-8
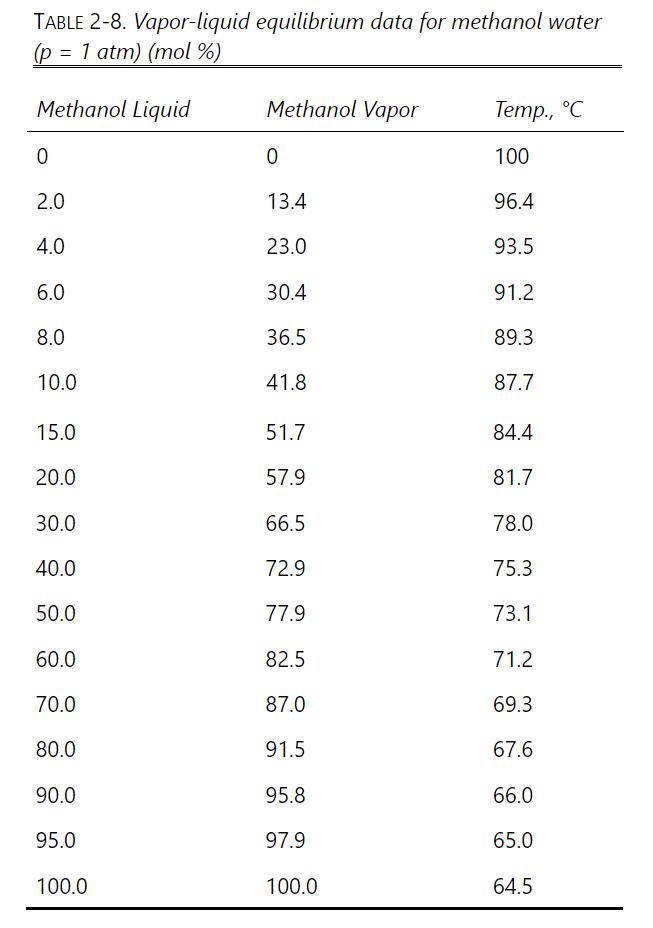
a. Find \(\mathrm{W}_{\text {final }}\), distillate \(\mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\) using Simpson's rule with one part.
b. Find \(\mathrm{W}_{\text {final }}\), distillate \(\mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\) using the Gaussian quadrature formula.
c. Explain why the area calculated by Simpson's rule is too high.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





