We are doing a single-stage, batch steam distillation of 1-octanol. The unit operates at (760 mathrm{~mm} mathrm{Hg}).
Question:
We are doing a single-stage, batch steam distillation of 1-octanol. The unit operates at \(760 \mathrm{~mm} \mathrm{Hg}\). The batch steam distillation is operated with liquid water present. The distillate vapor is condensed, and two immiscible liquid layers form. The feed is \(90.0 \mathrm{~mol} \%\) octanol, and the rest is nonvolatile organic compounds. The feed is \(1.0 \mathrm{kmol}\). We desire to recover \(95 \%\) of the octanol. The Antoine equation data are listed in Table 2-3 (note the units used).
Table 2-3
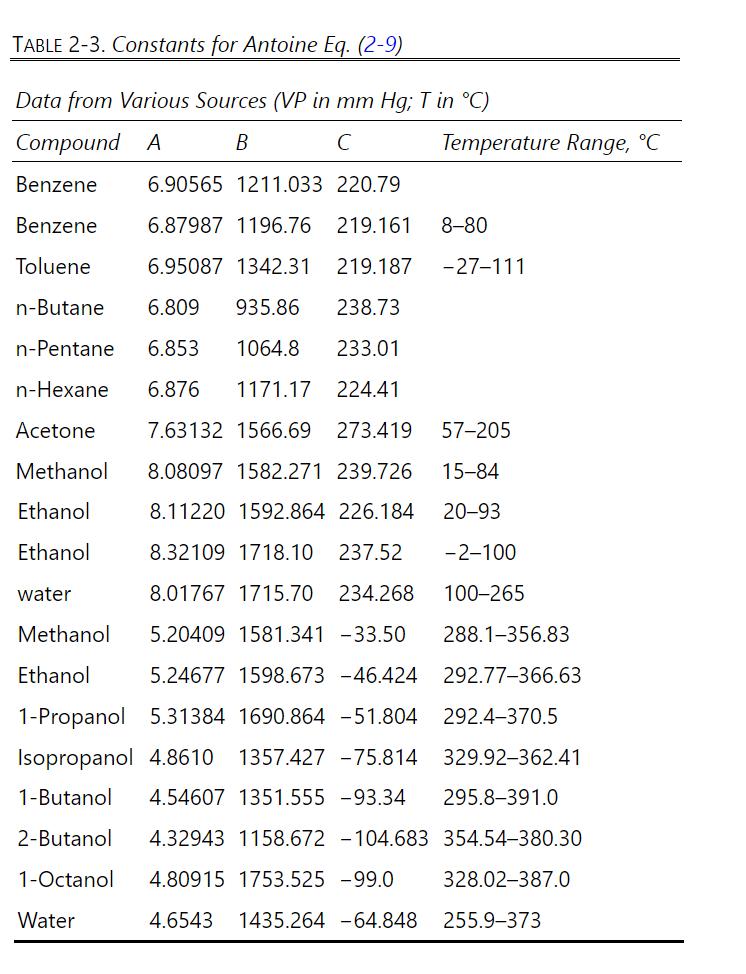
a. Find the operating temperature of the still at the beginning and end of the batch.
b. Find the amount of organics left in the still pot at the end of the batch.
c. Find the kmoles of octanol recovered in the distillate.
d. Find the kmoles of water condensed in the distillate product. To do this, use the average still temperature to estimate the average octanol vapor pressure, which can be assumed to be constant. To numerically integrate Eq. \((9-23 b)\), relate \(\mathrm{x}_{\text {org }}\) to \(\mathrm{n}_{\text {org }}\) with a mass balance around the batch still.
e. Compare with your answer to Problem 8.D11. Which system produces more water in the distillate? Why?
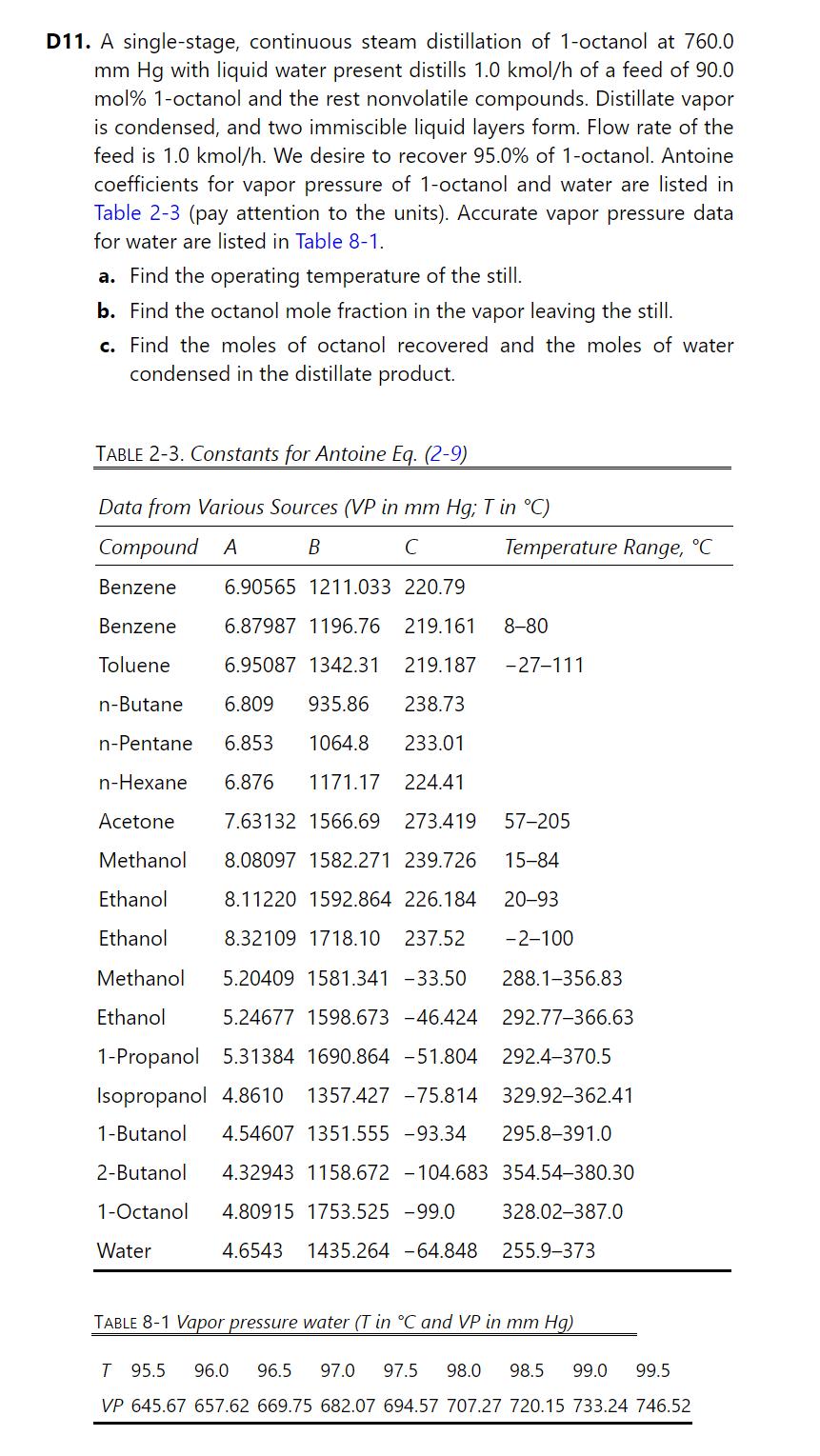
Problem 8.D11.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





