We wish to batch distill a mixture of 1-butanol and water. This system has a heterogeneous azeotrope
Question:
We wish to batch distill a mixture of 1-butanol and water. This system has a heterogeneous azeotrope (see Chapter 8), so we use the system shown in Figure 9-10. The bottom liquid layer from the liquid-liquid separator ( \(97.5 \mathrm{~mol} \%\) water) is removed as product, and the top liquid layer \((57.3 \mathrm{~mol} \%\) water) is returned to the still pot. Pressure is \(1 \mathrm{~atm}\). The feed is \(20.0 \mathrm{kmol}\) and \(40.0 \mathrm{~mol} \%\) water. Data are given in Problem 8.D2.
Figure 9-10
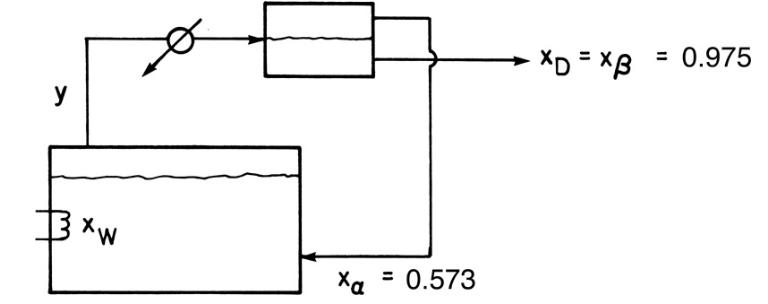
a. If the final concentration in the still pot is \(\mathrm{x}_{\mathrm{W} \text {,final }}=0.28\), find \(\mathrm{D}_{\text {total }}\) in kmoles.
b. If the batch distillation is continued past \(\mathrm{x}_{\mathrm{W} \text {,final }}=0.28\), what is the lowest value of \(\mathrm{x}_{\mathrm{W} \text {,final }}\) that can be obtained while still producing a distillate \(\mathrm{x}_{\mathrm{D}}=0.975\) ?
Problem 8.D2.
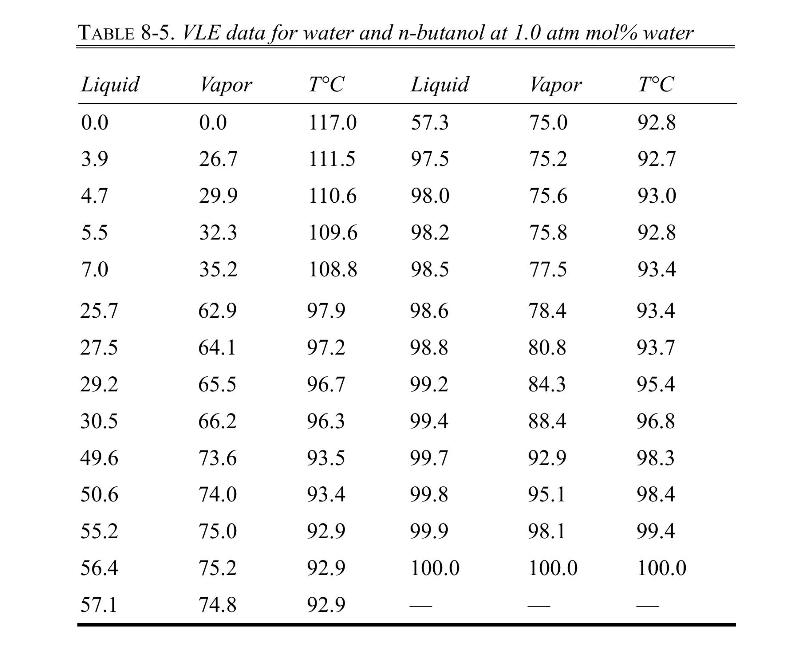
VLE data for water and n-butanol are given in Table 8-5. We have flash distillation systems separating \(100.0 \mathrm{kmol} / \mathrm{h}\) of two different water and \(\mathrm{n}-\) butanol mixtures.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





