The compound (mathrm{NO}_{2} mathrm{Cl}) is thought to decompose to (mathrm{NO}_{2}) and (mathrm{Cl}_{2}) by the following mechanism: Derive
Question:
The compound \(\mathrm{NO}_{2} \mathrm{Cl}\) is thought to decompose to \(\mathrm{NO}_{2}\) and \(\mathrm{Cl}_{2}\) by the following mechanism:
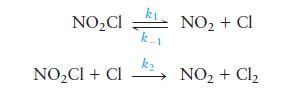
Derive the rate law for the production of \(\mathrm{Cl}_{2}\) using the steady-state approximation.
Transcribed Image Text:
NOCl NOCI+ CI k_1 NO + Cl NO + Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The steadystate approximation is a useful tool in chemical kinetics for simplifying reaction mechani...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Hydrogen radicals are important to sustaining combustion reactions. Consequently, if chemical compounds that can scavenge the hydrogen radicals are introduced, the flames can be extinguished. While...
-
One of the major reasons for engineoil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an antioxidant. Without an inhibitor to oxidation present,...
-
Titanium dioxide is a wide-bandgap semiconductor that is showing promise as an insulating dielectric in VLSI capacitors and for use in solar cells. Thin films of TiO2 are to be prepared by chemical...
-
Let Z[] be the following set of rational numbers { a Z, k N} (and recall that N = {0, 1, 2,...} in this class). Let the addition and multiplication for Z[] be the usual addition and multiplication...
-
Find an equation of the curve that satisfies dy/dx = 4x3y and whose -intercept is 7.
-
Given (a) Estimate the step size required to maintain stability using the explicit Euler method. (b) If y(0) = 0, use the implicit Euler to obtain a solution from t = 0 to 2 using a step size of 0.1....
-
Indicate whether the actions listed a through \(h\), primarily represent initiatives for cost containment (CC), cost avoidance (CA), or cost reduction (CR). Indicate what costs were contained,...
-
The bank reconciliation prepared by Gemma Tours on March 31, 2014, appeared as follows: The Cash account in the General Ledger appeared as follows on April 30: A list of deposits made and cheques...
-
Problem 10-21 Cost-Cutting Proposals [LO2] Tanaka Machine Shop is considering a four-year project to improve its production efficiency. Buying a new machine press for $465,000 is estimated to result...
-
The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation: Table 15.5 gives the results of four experiments involving this reaction. Using these...
-
Hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst \(\left(\mathrm{MnO}_{2}ight)\). The activation energy of the uncatalyzed reaction is \(70.0 \mathrm{~kJ} /...
-
Explain the term 'kiting'. Describe audit procedures that can be used to uncover this type of fraud.
-
The Qwik Market & Branding Company has found the following results of married people in a taste test of a popular high energy drink. Wife Prefers Wife Does Not Prefer Husband Prefers .08 .06 Husband...
-
12 Stiler XYZ currently has an enterprise value of $600 million, 20 million shares outstanding, $200 million in excess cash and no debt. Assuming XYZ uses its excess cash to repurchase shares, and...
-
A steel cable that weighs 8 lb/ft is used to pull a 500 lb block of concrete from the ground to the top of a 120 ft tall building. Let x be the distance, in feet, from the block to the TOP of the...
-
Simplify. -2v 3 3 4 Write your answer without parentheses.
-
Data for Barry Computer Co. and its industry averages follow. Barry Computer Company: Balance Sheet as of December 31, 2016 (In Thousands) Cash $58,320 Accounts payable $116,640 Receivables 196,830...
-
Why is it desired to have smooth, continuous flow on the shop floor?
-
When an electric field is applied to a shallow bath of vegetable oil, why do tiny bits of thread floating in the oil align with the field like compasses in a magnetic field?
-
Draw the Lewis structure and give the approximate bond angles of (a) C 2 H 4 ; (b) ClCN; (c) OPCl 3 ; (d) N 2 H 4 .
-
Hypochlorous acid, HClO, is found in white blood cells, where it helps to destroy bacteria. Draw two Lewis structures with different atom arrangements for HClO and select the most likely structure by...
-
The perchlorate ion, ClO 4 , is described by resonance structures. (a) Draw the Lewis structures that contribute to the resonance hybrid and identify the most plausible Lewis structures by using...
-
Suppose the average return on FTSE TMX Canada long-term bonds is 6.90% and the standard deviation is 8.10% and the average return and standard deviation on T-bills are 4.00% and 3.50%, respectively....
-
2 Investment X offers to pay you $5,300 per year for eight years, whereas Investment Y offers to pay you $7,300 per year for five years. Which of these cash flow streams has the higher present value...
-
Compare two cloud accounting systems. From the information available on the company websites, how do their reporting capabilities differ? From your own accounting knowledge, what are some useful...

Study smarter with the SolutionInn App


