Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11
Question:
Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11 in the textbook. Compare to the experimental values in Appendix B.
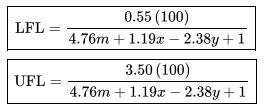
Data From Appendix B:
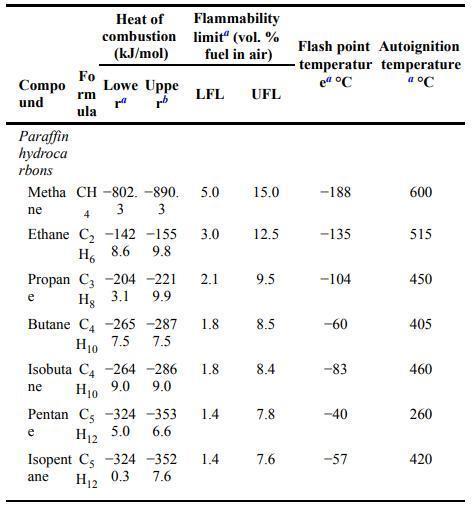
Equation 6-10 and 11:
Transcribed Image Text:
Paraffin hydroca rbons Fo rm ula Compo Lowe Uppe und Heat of combustion (kJ/mol) Flammability limit" (vol. % fuel in air) Metha CH-802. -890. 5.0 ne 3 3 e LFL 4 Ethane C -142 -155 3.0 H 8.6 9.8 Pentan C -324 -353 6.6 H12 5.0 Propan C3 204 -221 2.1 9.9 Hg 3.1 e Butane C4 -265-287 1.8 7.5 H10 7.5 Isobuta C4 -264 -286 1.8 8.4 ne H0 9.0 9.0 1.4 UFL Isopent C -324 -352 1.4 ane H12 0.3 7.6 15.0 12.5 9.5 8.5 7.8 7.6 Flash point Autoignition temperatur temperature el C a C -188 -135 -104 -60 -83 -40 -57 600 515 450 405 460 260 420
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To calculate the Lower Flammability Limit LFL and Upper Flammability Limit UFL for ethylene using the given equations we need to know the stoichiometr...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Estimate the upper and lower flammable limits for carbon monoxide and heptane using the stoichiometric method via Equations 6-10 and 6-11 in the text. Compare to experimental values provided in...
-
Molecular simulation can be used to explore the accuracy and significance of individual contributions to an equation of state. Use the DMD module at Etomica.org to explore Xes energy departure. (a)...
-
A certain apple bruises if a net force greater than 9 . 5 N is exerted on it . Would a 0 . 1 3 k g apple be likely to bruise if it falls 1 . 8 m and stops after sinking 0 . 0 5 m into the grass?...
-
Determine the volume of the solid generated by rotating the semi elliptical area shown about (a) The axis AA², (b) The axis BB², (c) The y axis.
-
Scout is the vice present for marketing at Sun Field Industries. She earns $140,000 annually and is paid on a semimonthly basis. As of October 31, Scout has year-to-date earnings of $116,666.67. The...
-
Mercury is about \(3.114 \times 10^{7}\) miles from the sun. Neptune is about \(2.781 \times 10^{9}\) miles from the sun. How many times further is Neptune from the sun than Mercury?
-
Jurassic Company owns machinery that cost $900,000 and has accumulated depreciation of $380,000. The expected future net cash flows from the use of the asset are expected to be $500,000. The fair...
-
Scenario Analysis and Portfolio Risk. The common stock of Bell Tower, a restaurant chain, will generate payoffs to investors next year, which depend on the state of the economy, as follows: ...
-
Estimate the LOC of ethylene using Equations 6-15 and 6-16 in the textbook. Compare to the experimental value in Table 6-3. Table 6-3: Equation 6-15: Equation 6-16: Gas or vapor Methane Ethane...
-
A gas cylinder contains a gas mixture composed of \(50 \%\) methane and \(50 \%\) ethylene by volume. Estimate the LFL and the UFL for this gas mixture. Compare to the experimental values of \(3.6...
-
Heat Q, which has the SI unit of joule (J), is the quantity in mechanical engineering that describes the transit of energy from one location to another. The equation for the flow of heat during the...
-
TASK PERFORMANCE Instructions: Determine the requirements for each of the independent cases. Show your computations (20 items x 5 points) Case 1 LOVE INC. purchased machinery on January 1, 201A, at...
-
Jean and Tom Perritz own and manage Happy Home Helpers, Inc. (HHH), a house-cleaning service. Each cleaning (cleaning one house one time) takes a team of three house cleaners about 1.5 hours. On...
-
This is all information I have and want to do some calculations, Venture capital (VC)investment Problem 1: Suppose a Venture capital (VC) investor wants to earn a 30% return on its investment and...
-
Production Report Analysis: Prepare production reports under the weighted average as well as the FIFO method. What is the cost per bottle under each method? What is considered as normal and abnormal...
-
Which of the three approaches (postpositivist, structuralist, and culturalist) do you most agree with and which are most effective and why? please provide an example of how one of these approaches...
-
1. Which of the following combinations correctly describes the relationship between foreign currency transactions, exchange rate changes, and foreign exchange gains and losses? .:. 2. In accounting...
-
Citing a scientific article, explain in your own words, how DNA fingerprinting has been used in forensic science to solve crimes and why it may not always be accurate or effective.
-
Referring to the description in Problem P3.16, if the viscosity of water is 0.01 poise, determine the value in terms of the units (a) Slug/(ft s) (b) kg/(m s). Problem P3.16 The property of a fluid...
-
The fuel efficiency of an aircrafts jet engines is described by the thrust-specific fuel consumption (TSFC). The TSFC measures the rate of fuel consumption (mass of fuel burned per unit time)...
-
An automobile engine is advertised as producing a peak power of 118 hp (at an engine speed of 4000 rpm) and a peak torque of 186 ft lb (at 2500 rpm). Express those performance ratings in the SI...
-
1. Try running the DebuggerExercise.java file. You should see the following message in the console window at the bottom of the screen: Congratulations! You've found a way out of your labyrinth. ...
-
please help me fix my code. thanks very much. Define a SmallArray class that holds a small array of T elements and a pointer to the next SmallArray object. Add a size parameter to the ListArray...
-
Q. Let's say we add a new for statement grammar to the new programming language S as follows. For the program implemented in the following language S, indicate the result of state transition...

Study smarter with the SolutionInn App


