For the following, mix equal volumes of one solution from Group I with one solution from Group
Question:
For the following, mix equal volumes of one solution from Group I with one solution from Group II to achieve the indicated pH. Calculate the pH of each solution.
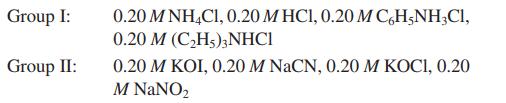
a. The solution with the lowest pH
b. The solution with the highest pH
c. The solution with the pH closest to 7.00
Transcribed Image Text:
Group I: Group II: 0.20 M NH4C1, 0.20 M HC1, 0.20 M C6H5NH₂Cl, 0.20 M (C₂H5)3NHC1 0.20 M KOI, 0.20 M NaCN, 0.20 M KOCI, 0.20 M NaNO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a The solution with the lowest pH The solution with the lowest pH will be the one formed by mixing NH4Cl from Group I with HCl from Group II When NH4C...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
What error in this program needs to be fixed? def sum(num1, num2): return num1 + num2 5.5, 6)) print(sum(4,
-
Consider the pooled t variable Tp from part (b) of the previous exercise. a. Use this t variable to obtain a pooled t confidence interval formula for 1 2 . b. The article Effect of Welding on a...
-
If there was any ambiguity on the application, should it be resolved in favor of the insured or the insurer? Provident Insurance, Inc., issued an insurance policy to a company providing an employee,...
-
Hardy Companys cost of goods sold is consistently 60% of sales. The company plans to carry ending merchandise inventory for each month equal to 20% of the next months budgeted cost of goods sold. All...
-
Cost Technology describes itself as a "global consulting company specializing in profit management." The company helps manufacturing, service, and government organizations implement methods, such as...
-
The physically realizable form of the PD transfer function is given in the first equation of Exercise 8.1.(a) Show how to obtain this transfer function with a parallel arrangement of two much simpler...
-
Construction work authorized amounted to $600,000. As a part of this, a contract for $550,000 was signed with a private firm; the remainder of the work was to be done by water utility employees....
-
Shauna Coleman is single. She works as an architectural designer for Streamline Design (SD). Shauna wanted to determine her taxable income. She correctly calculated her AGI. However, she wasn't sure...
-
A 0.100-g sample of the weak acid HA (molar mass = 100.0 g/mol) is dissolved in 500.0 g water. The freezing point of the resulting solution is -0.0056C. Calculate the value of K a for this acid....
-
Calculate the pH of a 0.10-M solution of sodium phosphate. (See Exercise 183.) Data in Exercise 183 Consider the species PO 4 3- , HPO 4 2- , and H 2 PO 4 - . Each ion can act as a base in water....
-
Draftspeople often use the method shown in the sketch to draw an ellipse. Why does this method work?
-
The following figure shows a homogeneous cylinder of radius R and mass m that is free to rotate about its axis of rotation and that is connected to the wall through a spring. Assuming that the...
-
What ethical considerations arise in the implementation of stress management programs within organizational contexts, particularly concerning issues of confidentiality, privacy, and the stigma...
-
4. (14 points) Two source charges are located as shown in the figure. Point P is located 2 cm directly in the +y direction from q as shown. Point P2 is above q, closer than 2 cm, but the exact...
-
How to fix the application error ? describe the steps and procedure you would follow to fix the errors
-
How do change management methodologies such as Kotter's eight-step model, Lewin's three-step model, and Prosci's ADKAR framework provide structured approaches for planning, executing, and evaluating...
-
In a recent General Social Survey, respondents answered the question, In the past month, about how many hours have you spent praying, meditating, reading religious books, listening to religious...
-
A bar of a steel alloy that exhibits the stress-strain behavior shown in Figure 6.22 is subjected to a tensile load; the specimen is 375 mm (14.8 in.) long and has a square cross section 5.5 mm (0.22...
-
Diethyl malonate (the starting material for the malonic ester synthesis) reacts with bromine in acid-catalyzed conditions to form a product with molecular formula C 7 H 11 BrO 4 . (a) Draw the...
-
Cinnamaldehyde is one of the primary constituents of cinnamon oil and contributes significantly to the odor of cinnamon. Starting with benzaldehyde and using any other necessary reagents, show how...
-
Draw the condensation product that is expected when each of the following esters is treated with sodium ethoxide followed by acid workup. (a) (b) (c) OEt OEt
-
Camby Corp. can make one of the following four products: Product 1 Product 2 Product 3 Product 4 Selling price per unit $94.00 $211.00 $291.00 $42.00 Variable cost per unit $35.00 $117.00 $207.00...
-
Gastow Pumps is a manufacturer of commercial and heavy industrial Pumps. The firm's two product lines are called Directlift and Gravity. The primary raw materials are flexible steel sheets, and 23cm...
-
Caso de negocio: RoboTech. Irrumpiendo en el mercado estadounidense Con la informacin que Chen tena disponible en 2009, crees que la decisin de invertir 45 millones de dlares para diversificar...

Study smarter with the SolutionInn App


