Determine the shape of the following molecules using VSEPR theory. (a) SF 4 , (b) BrF 5
Question:
Determine the shape of the following molecules using VSEPR theory.
(a) SF4,
(b) BrF5
Strategy As always, we start by drawing the Lewis structures. Then count the number of electron pairs around the central atom and determine the spatial arrangement of electron pairs, consulting Table 7.3 as necessary. Place the lone pairs in positions where the electron repulsions are minimized and describe the resulting geometric arrangement of the atoms.
Table 7.3
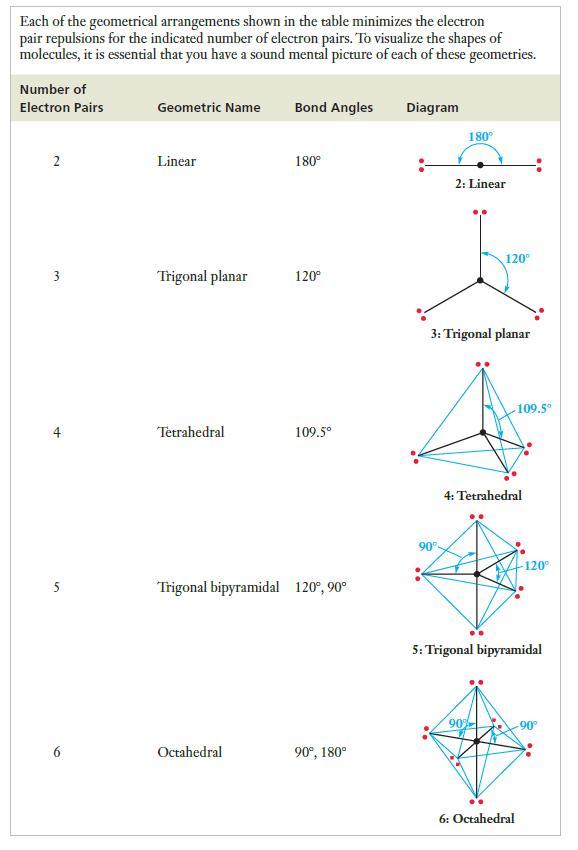
Transcribed Image Text:
Each of the geometrical arrangements shown in the table minimizes the electron pair repulsions for the indicated number of electron pairs. To visualize the shapes of molecules, it is essential that you have a sound mental picture of each of these geometries. Number of Electron Pairs 2 m t 5 6 Geometric Name Linear Trigonal planar Tetrahedral Bond Angles Octahedral 180° 120° 109.5° Trigonal bipyramidal 120°, 90° 90⁰, 180° Diagram 180° 90° 2: Linear 3: Trigonal planar 120⁰ 4: Tetrahedral 90% 109.5⁰ 5: Trigonal bipyramidal 6: Octahedral 120° -90°
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The Lewis structure of SF 4 is as shown There are five pairs of electrons around sulfur so the shape with minimum interaction is a trigonal bipyrami...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Use VSEPR theory to determine the shape of the NOF molecule. Strategy Once again, we start by drawing the Lewis structure. Then count the number of regions of electron density around the central...
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
For the instances mentioned below, identify the applicable laws/regulators. (Indicate multiple regulators, where applicable) a) Amalgamation of a weak private bank with another foreign private bank...
-
For problems involving composite bodies composed of two or more materials, the elasticity solution requires both boundary conditions and interface conditions between each material system. The...
-
Can activities 5 and 6 of Figure be eliminated? What risks does a project manager incur if these activities areeliminated? CONTRACTOR PROGRAM OFFICE REQUEST FOR HEDULES REVEL ROLIGH VERFY THAT ALL...
-
Sleep duration data (in hours/ night) are shown for the patients of Exercise 18.13. Sequence 1 received the investigational drug first and the placebo second; the reverse order applied to sequence 2....
-
Discuss why some form of theoretical pricing model is required for inventory valuation purposes.
-
1. Now that the new ESIP system is operational, Jane Rossman wants you to track system performance using various measurements. At a minimum, she expects you to monitor operational costs, maintenance...
-
x+5 6x-30 3. These two angles are 4. Set up an equation to solve these equation Use button to input the equation Input your answer WITHOUT spaces 5. Solve for x x= So, to solve for x we must set...
-
Although much less common than silicon devices, germanium-based semiconductors can also be fabricated. Which kind of material (n- or p-type) would result if pure germanium were doped with (a)...
-
Poly(vinyl alcohol) is used in several biomaterials applications, including surgical sutures. It is also used in drops for dry eyes and some contact lens solutions. Draw the Lewis structure of vinyl...
-
Morris Zapp and Philip Swallow consume wine and books. Morris has an initial endowment of 60 books and 10 bottles of wine. Philip has an initial endowment of 20 books and 30 bottles of wine. They...
-
Units Determine the cost assigned to ending inventory and to cost of goods sold using FIFO. Date Activities Units Acquired at Cost Total Sales Units sold at Retail Selling price per Cost Total cost...
-
How many new shares did your contribution buy? Image transcription text 2. On Feb 15, if EIF costs $40/share. a. How many new shares did your contribution buy? b. How many total shares do you now...
-
A bullet flying horizontally with a speed of 7 5 0 m / s bounces off a tank armor which is sloped at 4 5 degrees. Assuming that the collision is perfectly elastic and there is no friction, calculate...
-
After a tornado, a 0.50 g straw was found embedded 2.9 cm into the trunk of a tree. Part A If the average force exerted on the straw by the tree was 60 N, what was the speed of the straw when it hit...
-
The salespeople at Buffalo, a notebook manufacturer, commonly pressured operations managers to keep costs down so the company could give bigger discounts to large customers. John, the operations...
-
Explain why a financial asset can be viewed as a package of zero-coupon instruments.
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
A weak acid has a dissociation constant of K a = 2.50 10 2 . a. Calculate the degree of dissociation for a 0.093m solution of this acid using the DebyeHckel limiting law. b. Calculate the degree of...
-
Calculate the mean ionic activity of a 0.0350 m Na 3 PO 4 solution for which the mean activity coefficient is 0.685.
-
At 25C, the equilibrium constant for the dissociation of acetic acid, K a , is 1.75 10 5 . Using the DebyeHckel limiting law, calculate the degree of dissociation in 0.150 m and 1.50 m solutions...
-
Cash Budget with Supporting Cash Collections and Disbursements Schedules - Excel FILE HOME INSERT PAGE LAYOUT Calibri -11 A Paste BIU Clipboard Font FORMULAS DATA REVIEW VIEW % Sign In M Editing...
-
Hitzu Company sold a copier (that costs $3,500) for $7,000 cash with a two-year parts warranty to a customer on August 16 of Year 1. Hitzu expects warranty costs to be 5% of dollar sales. It records...
-
On 31 December 20X7, a company has the following bond on the statement of financial position: Bond payable, 7%, interest due semi-annually on 31 Dec. and 30 June; maturity date, 30 June 20x11 Premium...

Study smarter with the SolutionInn App


