A sample of americium oxide, AmO 2 , was prepared for use in a smoke detector, as
Question:
A sample of americium oxide, AmO2, was prepared for use in a smoke detector, as described in the introduction to this chapter. The detector contains 0.000330 g Am-242 oxide , and the absolute disintegration rate is measured at 3.70 × 107 disintegrations/s. Calculate the half-life of 241Am, and express the answer in years.
Strategy Equation 21.1 relates the decay rate to the number of atoms present, which can be calculated from the mass of AmO2 and Avogadro’s number.
Equation 21.1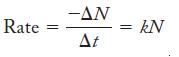
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





