In 1911, Ernest Rutherford discovered the basic structure of the atom by shooting positively charged alpha particles
Question:
In 1911, Ernest Rutherford discovered the basic structure of the atom by "shooting" positively charged alpha particles with a speed of 107 m per sec at a piece of gold foil 6 × 10-7 m thick. Only a small percentage of the alpha particles struck a gold nucleus head-on and were deflected directly back toward their source. The rest of the particles often followed a hyperbolic trajectory because they were repelled by positively charged gold nuclei. As a result of this famous experiment, Rutherford proposed that the atom was composed mostly of empty space with a small and dense nucleus.
The figure shows an alpha particle A initially approaching a gold nucleus N and being deflected at an angle u = 90°. N is located at a focus of the hyperbola, and the trajectory of A passes through a vertex of the hyperbola.

(a) Determine the equation of the trajectory of the alpha particle if d = 5 × 10-14 m.
(b) What was the minimum distance between the centers of the alpha particle and the gold nucleus? Write the answer using scientific notation. Round to the nearest tenth.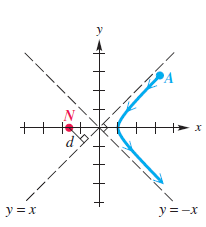
Step by Step Answer:

College Algebra
ISBN: 978-0134697024
12th edition
Authors: Margaret L. Lial, John Hornsby, David I. Schneider, Callie Daniels





