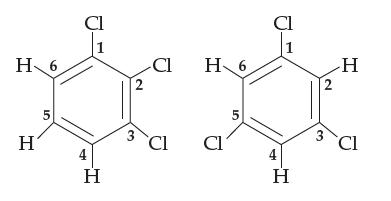
Consider 1,2,3-trichlorobenzene and 1,3,5-trichlorobenzene, both shown below (the carbon atoms are numbered as shown). Both are flat
Question:
Consider 1,2,3-trichlorobenzene and 1,3,5-trichlorobenzene, both shown below (the carbon atoms are numbered as shown). Both are flat molecules with 120° bond angles, yet one of them is polar and the other nonpolar. Which is which, and why? For the polar one, indicate which part of the molecule is δ+ and which is δ–.
Transcribed Image Text:
H 6 H 5 Cl 1 H 2 3 -Cl Cl H 6 Cl 5 Cl H 2 3 H Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
123trichlorobenzene is polar while 135trichlorobenzene is nonpolar To determine the polarity of a mo...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A local manufacturing company produces heating and colling appliances for home and industrial applications. These include refrigerators, water coolers, water heaters, air conditioners, sandwich...
-
Consider the tensile stress-strain diagrams in Figure 6-28 labeled 1 and 2 and answer the following questions. These diagrams are typical of metals. Consider each part as a separate question that has...
-
For each of the following organic molecules draw a Lewis structure in which the carbon atoms are bonded to each other by single bonds: (a) C2H6, (b) C4H10, (c) C5H12. For (b) and (c), show only...
-
The OrbitTrack Company specializes in developing and selling a wide range of high-quality scooters. Sales representatives report that there is a growing demand for racing scooters. OrbitTrack's...
-
Spaulding Manufacturing Company has determined the cost of manufacturing a unit of product as follows, based on normal production of 100,000 units per year: Direct materials . . . . . . . . . . . . ....
-
Describe which economic role of government (allocation, distribution, or stabilization) is addressed in each of the following cases of government agencies or programs: a. Environmental Protection...
-
How do you explain the fact that rates of return required by investors may be identical for two groups of totally different activities (oil and IT services for example) as long as they have the same .
-
A debt of $5000 can be repaid, with interest at 8%, by the following payments. Year Payment 1 $ 500 2 1000 3 1500 4 2000 5 X The payment at the end of the fifth year is shown as X. How much is X?
-
A. 9 E. F.
-
Cyclohexane, formula C 6 H 12 , is often drawn as a puckered (not flat) ring as shown below (the purple and blue atoms are the hydrogens). Explain how drawing it as a puckered ring makes it...
-
There are exceptions to the predictions of VSEPR. Consider CH 3 , known as a methyl radical. (a) Create a dot diagram for the methyl radical. How is it fundamentally different from other dot diagrams...
-
From the following information, compute the early, late, and slack times for each activity. Identify the critical path. Code Software Lag 2 A Debug Lag 2 Design B D Order Assemble Install Parts Lag 3...
-
What are the implications of transformational leadership on employee engagement and innovation, and how can leaders balance transformational and transactional styles for optimal organizational...
-
For each of the following, you must show all necessary steps to receive full credit (where appropriate). This HW is worth 10 points. Not all problems may be graded. 1. possible: a) Three married...
-
What are the ethical implications of using behavioral economics principles to influence employee decision-making and productivity within organizations ?
-
As a Marketer for West Pack, you want to design a customer driven marketing strategy. You complete an audit to select the customers to serve and value proposition to include. Look at the options and...
-
Sometime in the past, Taxpayer A loaned Taxpayer B $100,000. However, no one can find the written debt instrument. Taxpayer B acknowledges that the debt exists, but given its possible...
-
Recent attention has been given to the percentage of successful free throws that resulted in points made in mens college basketball and the number of dribbles the shooter takes prior to the free...
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
Find the result of operating with (1/r 2 ) (d /dr) (r 2 d /dr) + 2/r on the function Ae br . What must the values of A and b be to make this function an eigenfunction of the operator?
-
Normalize the set of functions n (θ) = e inθ , 0 ¤ θ ¤ 2Ï. To do so, you need to multiply the functions by a so-called normalization constant...
-
Show that the set of functions n () = e in , 0 2is orthogonal if n is an integer. To do so, you need to 2 0 * m () n () = d = 0 for m n if n and m are intergers.
-
[STAT6032 ONLY] Use the Bonferroni method to compare the following treatments (i.e. these four specific level combinations of speed and depth): (2,3), (2,4), (3,3) 6%). Calculate the critical...
-
Provide a purchase journal entry of a product (50$ above), which is comprised of a rational cognition purchase decision, extensive problem-solving decision, ideal state opportunity recognition,...
-
12345 1 Income Statement 2021 2020 2019 2018 Period Ending: 30/06 30/06 30/06 30/06 5 Total Revenue 671.74 598.85 784.51 777.95 6 Revenue 671.74 598.85 784.51 777.95 7 Other Revenue, Total 8 Cost of...

Study smarter with the SolutionInn App


