Which nitrogen atom in imidazole (Problem 12.16) is more basic according to the following electrostatic potential map?
Question:
Which nitrogen atom in imidazole (Problem 12.16) is more basic according to the following electrostatic potential map? Why?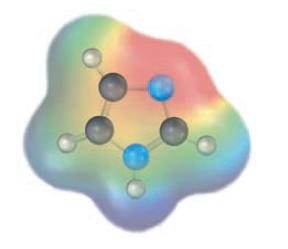
Problem 12.16
The five-membered heterocycle imidazole contains two nitrogen atoms, one “pyrrole-like” and one “pyridine-like.” Draw an orbital picture of imidazole, and indicate the orbital in which each nitrogen has its electron lone pair.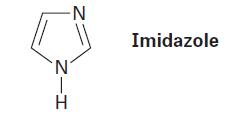
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





