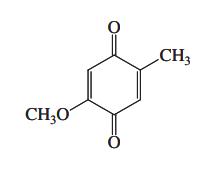
Diels-Alder cycloaddition of 1,3-butadiene with the cyclic dienophile shown in the margin takes place at only one
Question:
Diels-Alder cycloaddition of 1,3-butadiene with the cyclic dienophile shown in the margin takes place at only one of the two carbon – carbon double bonds in the latter to give a single product. Give its structure and explain your answer. Watch stereochemistry. This transformation was the initial step in the total synthesis of cholesterol, completed by R. B. Woodward in 1951. This achievement, monumental for its time, revolutionized synthetic organic chemistry.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





