Melamine, or 2,4,6-triaminotriazine, is a toxic heterocyclic compound that has been implicated in the illnesses and deaths
Question:
Melamine, or 2,4,6-triaminotriazine, is a toxic heterocyclic compound that has been implicated in the illnesses and deaths of both house pets and humans who ingested melamine-contaminated food. As its formula shows, melamine has a very high nitrogen content. Proteins (Chapter 26) are the main natural source of nitrogen in foods, and nitrogen analysis is commonly used to determine protein content in foods. The illegal addition of melamine to packaged foods increases their nitrogen content and makes them appear richer in protein; in reality, they are deadly.
(a) A typical protein in food contains about 15% nitrogen. What is the % N in melamine? Does this result explain the motivation behind the melamine “doping” of packaged foods?
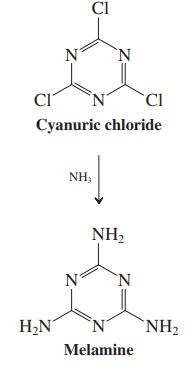
(b) Melamine is synthesized by addition of ammonia to cyanuric chloride (2,4,6-trichlorotriazine, see equation in the margin). What kind of reaction is this? Formulate a mechanism and explain why cyanuric chloride displays this type of chemistry.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





