Propanal are isomers with the formula C 3 H 6 O. The heat of combustion of propanal
Question:
Propanal
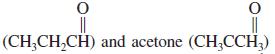
are isomers with the formula C3H6O. The heat of combustion of propanal is - 434.1 kcal mol-1, that of acetone -427.9 kcal mol-1.
(a) Write a balanced equation for the combustion of either compound.
(b) What is the energy difference between propanal and acetone? Which has the lower energy content?
(c) Which substance is more thermodynamically stable, propanal or acetone?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





