Reductive amination of excess formaldehyde with a primary amine leads to the formation of a dimethylated tertiary
Question:
Reductive amination of excess formaldehyde with a primary amine leads to the formation of a dimethylated tertiary amine as the product (see the following example). Propose an explanation.
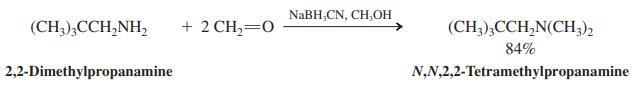
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:




