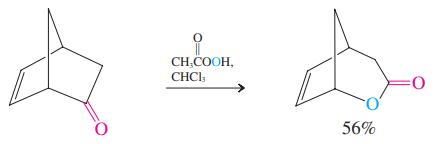
The general equation for the Baeyer-Villiger oxidation (see p. 808) begins with a reaction between a ketone
Question:
The general equation for the Baeyer-Villiger oxidation (see p. 808) begins with a reaction between a ketone and a peroxycarboxylic acid to form a peroxy analog of a hemiacetal. Formulate a detailed mechanism for this process.
Data From Page 808
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





