A water cleanup is stripping vinyl chloride from contaminated ground water at (25.0^{circ} mathrm{C}) and (850.0 mathrm{~mm}
Question:
A water cleanup is stripping vinyl chloride from contaminated ground water at \(25.0^{\circ} \mathrm{C}\) and \(850.0 \mathrm{~mm} \mathrm{Hg}\) using a countercurrent, staged stripper. Feed is 5.0 ppm (molar) vinyl chloride. Outlet water that contains \(0.1 \mathrm{ppm}\) (molar) vinyl chloride is required. Inlet air used for stripping is pure. For a liquid flow rate of \(\mathrm{L}=1.0 \mathrm{kmol} / \mathrm{h}\), determine the following:
a. Minimum gas flow rate \(\mathrm{G}_{\text {min }}\) in \(\mathrm{kmol} / \mathrm{h}\).
b. If \(\mathrm{G}=2.0 \mathrm{G}_{\text {min }}\), use a McCabe-Thiele diagram to determine the number of equilibrium stages needed (including a fractional number). Note: Scales on your \(\mathrm{y}\) and \(\mathrm{x}\) axes should be different. Calculate two points to plot the straight lines.
c. If \(\mathrm{G}=2.0 \mathrm{G}_{\mathrm{min}}\), use one form of the Kremser equation to determine the number of equilibrium stages needed (including a fractional number).
d. For parts \(\mathrm{b}\) and \(\mathrm{c}\), what is the concentration of vinyl chloride in outlet gas? What would you propose doing with this gas (it cannot be vented to the atmosphere)
e. Are the assumptions required for the solution methods satisfied?
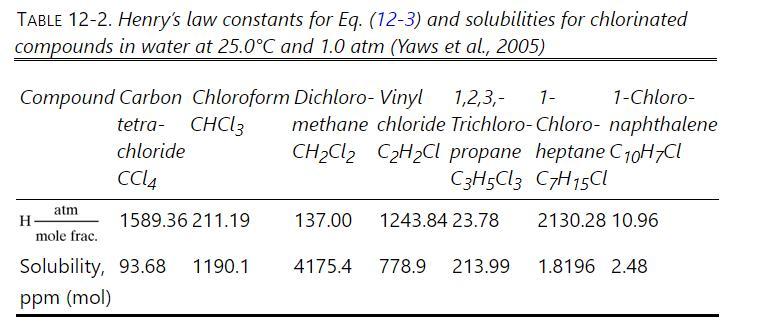
Data are in Table 12-2.
Table 12-2
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat




