Absorb ammonia from air into water at (20.0^{circ} mathrm{C}) and (1.5 mathrm{~atm}) pressure. Inlet water is recycled
Question:
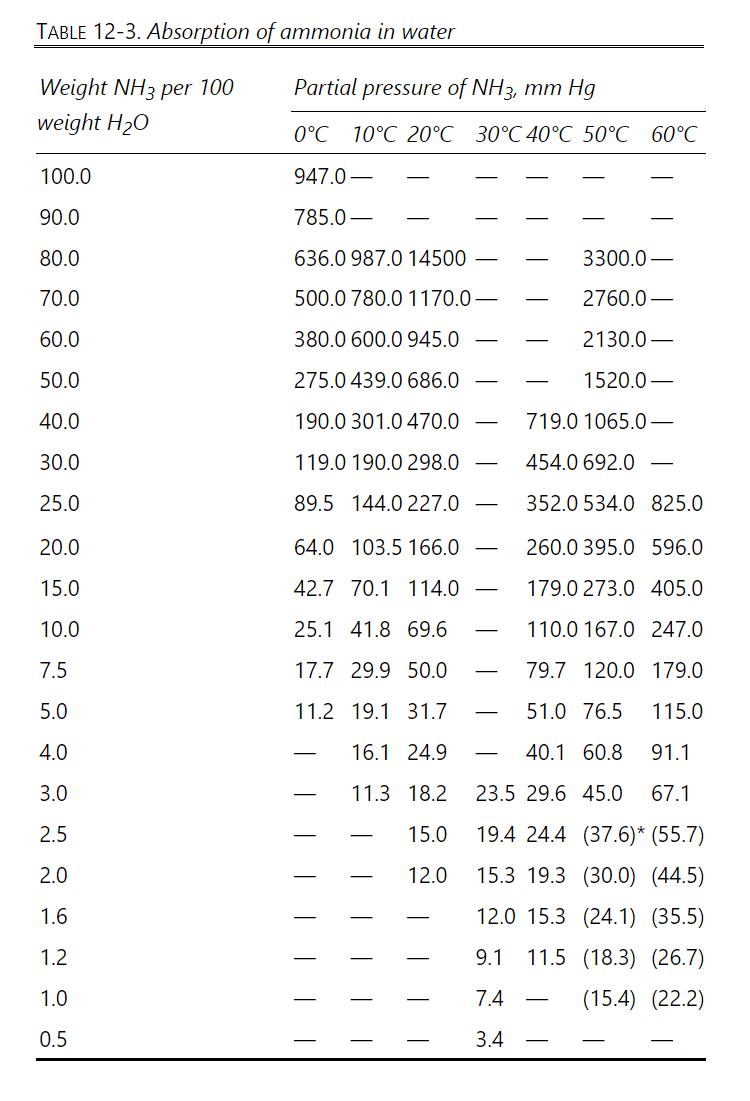
Absorb ammonia from air into water at \(20.0^{\circ} \mathrm{C}\) and \(1.5 \mathrm{~atm}\) pressure. Inlet water is recycled from a stripper and contains \(0.2 \mathrm{wt} \%\) ammonia. Gas flow rate is \(100.0 \mathrm{kmol} / \mathrm{h}\). Inlet gas is \(3.1 \mathrm{~mol} \%\) ammonia. Exit gas should be 0.31 \(\mathrm{mol} \%\) ammonia. Assume L/V is constant. Equilibrium data are in Table 12-3.
a. What is the minimum flow rate of water in \(\mathrm{kg} / \mathrm{h}\) ?
b. What is the exit concentration of the liquid, and how many equilibrium stages are needed if \(\mathrm{L}=1.5 \times \mathrm{L}_{\text {min }}\) ?
Keep your units straight. In Table 12-3, 10.0 weight \(\mathrm{NH}_{3}\) per 100.0 weight water \(=10.0\) weight \(/ 110.0\) weight solution \(=0.090909\) mass fraction.
Approach 1: Convert all units to either mass or to moles. [MW \(\mathrm{NH}_{3}=\) 17.0, \(\mathrm{MW} \mathrm{H}_{2} \mathrm{O}=18.0\), \(\mathrm{MW}\) air \(\left.=28.9\right]\)
Approach 2: Keep liquid in weight fraction and gas in mole fraction, and plot equilibrium in this form. In the \(\mathrm{NH}_{3}\) mass balance, if \(\mathrm{y}\) is mole fraction \(\mathrm{NH}_{3}\) in gas and \(\mathrm{x}\) is weight fraction ammonia in liquid, \(\mathrm{Vy}=\) \((\mathrm{kmol}\) gas \(/ \mathrm{h})\left(\mathrm{kmol} \mathrm{NH} \mathrm{N}_{3} / \mathrm{kmol}\right.\) gas \()=\mathrm{kmol} \mathrm{NH}_{3} / \mathrm{h}\), and \(\mathrm{Lx}=(\mathrm{kg}\) liquid \(/ \mathrm{h})\) \(\left(\mathrm{kg} \mathrm{NH}_{3} / \mathrm{kg}\right.\) liquid \()=\mathrm{kg} \mathrm{NH} / \mathrm{h}\).
Then adjust the \(\mathrm{NH}_{3}\) mass balance with the molecular weight of \(\mathrm{NH}_{3}\) so that all terms are in \(\mathrm{kg} \mathrm{NH}_{3} /\) h. Derive the operating equation from this form of mass balance.
c. Estimate the overall efficiency from the O'Connell correlation, and estimate the number of actual stages required.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat




