An equilibrium mixture of ethylene and propylene is at (2500 mathrm{kPa}) and (25.0^{circ} mathrm{C}). Find the vapor
Question:
An equilibrium mixture of ethylene and propylene is at \(2500 \mathrm{kPa}\) and \(25.0^{\circ} \mathrm{C}\). Find the vapor and liquid mole fractions of ethylene. Use DePriester charts or Eq. (2-28). This is not a guess-and-check problem.
Equations (2-28)
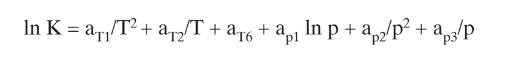
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





