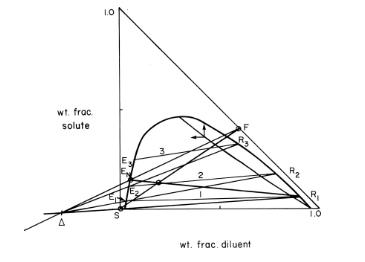
For Figure 13-24, suppose the raffinate concentration had to be obtained with exactly two equilibrium stages. This
Question:
For Figure 13-24, suppose the raffinate concentration had to be obtained with exactly two equilibrium stages. This can be accomplished by changing amount of solvent used. Would we want to increase or decrease amount of solvent? Explain the effect this change will have on \(\mathrm{M}, \mathrm{E}_{\mathrm{N}}, \Delta\), and number of stages required.
Figure 13-24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





