Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written.
Question:
Calculate E°cell for each balanced redox reaction and determine if the reaction is spontaneous as written.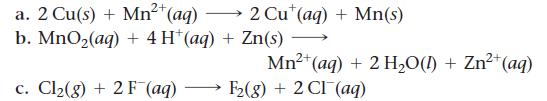
Transcribed Image Text:
2+ a. 2 Cu(s) + Mn²+ (aq) b. MnO₂(aq) + 4H+ (aq) + Zn(s) c. Cl₂(g) + 2 F (aq) 2 Cut (aq) + Mn(s) 2+ Mn²+ (aq) + 2 H₂O(1) + Zn²+ (aq) F₂(g) + 2 CI (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 170 V nonsp...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written. a. O(g) + 2 HO(1) + 4 Ag(s) b. Br(1) 21 (aq) c. PbO (s) + 4H+ (aq) + Sn(s) 4 OH(aq) + 4 Ag+...
-
Toplob Inc. ("Toplob") provides employment consulting services. These services range from maintaining payroll records to taxation services, as well as general business advisory and consulting to new...
-
Which 3 accounts can't be merged in the chart of accounts?
-
At the beginning of last year, Diekow Productions set variable overhead standards of 10 machine hours at a rate of $ 10 per hour for each product produced. During the year, 10,800 machine hours were...
-
Jiminez Skill Shops Limited (JSSL) acquired inventory of $5,000 with terms of 1/5 n30. JSSL maintains access to a line of credit at a bank at 12% interest. Required: Should JSSL use its line of...
-
Presented below are summary financial data from the The Coca-Cola Company 2015 annual report. Using the ratio definitions from Exhibit 4.6, calculate the following liquidity and solvency ratios: cash...
-
Presented below is a major portion of Starbucks Note 4 to its fiscal 2008 Consolidated Financial Statements in which it describes its minority passive investments. Required a. As of its September 28,...
-
Operating cash flow. Grady Precision Measurement Tools has forecasted the following sales and costs for a new GPS system: annual sales of 40,000 units at $23 a unit, production costs at 38% of sales...
-
Determine whether or not each metal dissolves in 1 M HIO 3 . For those metals that do dissolve, write a balanced redox equation for the reaction that occurs. a. Au b. Cr
-
Determine whether or not each metal dissolves in 1 M HNO 3 . For those metals that do dissolve, write a balanced redox reaction showing what happens when the metal dissolves. a. Cu b. Au
-
A random sample of 10 shipments of stick-on labels showed the following order sizes. (a) Construct a 95 percent confidence interval for the true mean order size. (b) How could the confidence interval...
-
Describe the concepts, principles and constraints underlying financial statements.
-
What form of representation would you recommend for this new market or would you consider setting up a manufacturing subsidiary? Give reasons for your decision.
-
Soon after the precipitous decline of Arizonas real estate market in 1989, BFA management decided to establish a number of related affiliates. These affiliates were controlled by individuals with...
-
Distinguish between the effect on a CPA firms practice of enforcing the rules of conduct by the AICPA versus a state Board of Accountancy.
-
What, if any, further research needs to be undertaken before attempting to export to the United States?
-
Kate Royer is the new controller for ED Software, which develops and sells educational software. Shortly before the December 31 fiscal year-end, Justin Torabi, the company president, asks Royer how...
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
Imagine that we have Youngs Experiment, where one of the two pinholes is now covered by a neutral-density filter that cuts the irradiance by a factor of 10, and the other hole is covered by a...
-
Show (for normally incident plane waves) that if an aperture has a center of symmetry (i.e., if the aperture function is even), then the diffracted field in the Fraunhofer case also possesses a...
-
Suppose a given aperture produces a Fraunhofer field pattern E(Y, Z). Show that if the apertures dimensions are altered such that the aperture function goes from A(y, z) to A(αy,...
-
Xiaoxiao Corp. offers 7 percent coupon bonds with semiannual payments and a yield to maturity of 5.6 percent. The bonds mature in 13 years. What is the market price per bond if the face value is...
-
The composition of the Fingroup Fund portfolio is as follows: Stock Shares A 200,000 Price $ 39 B 272,000 50 414,000 34 D 540,000 35 The fund has not borrowed any funds, but its accrued management...
-
Consider the following information on large - company stocks for a period of years. Arithmetic Mean Large - company stocks 1 2 . 7 % Inflation 3 . 3 a What was the arithmetic average annual return on...

Study smarter with the SolutionInn App


