Write the equilibrium expression (K) for each of the following gas-phase reactions. a. N(g) + O(g)=2NO(g) b.
Question:
Write the equilibrium expression (K) for each of the following gas-phase reactions.
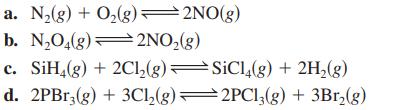
Transcribed Image Text:
a. N₂(g) + O₂(g)=2NO(g) b. N₂O₂(g)2NO₂(g) c. SiH4(g) + 2Cl₂(g) d. 2PBr3(g) + 3Cl₂(g) SiCl4(g) + 2H₂(g) 2PC13(g) + 3Br₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Consider a gas phase reaction where N2O4 (g) 2NO2 (g) at 25C. The equilibrium constant (Kp) for the reaction is 0.25 atm. a) Write the equilibrium expression for Kp. b) Calculate the partial...
-
A stirred tank with volume V t? (L) is charged with V 1 (L) of a liquid. B. The space above the liquid (volume V g = V t ? V 1 ) is filled with a pure gas, A, at an initial pressure P 0 (atm). The...
-
Give the expression for K for each of the following reactions. a. b. c. CaCO (s)CaO(s) CO2(g) Pbl2(s) H (a) HCO (aq)H2O) CO2(g)
-
A slipper-pad bearing (Fig. P1023) is often encountered in lubrication problems. Oil flows between two blocks; the upper one is stationary, and the lower one is moving in this case. The drawing is...
-
What If the Facts Were Different? Suppose that shortly before Pallisters death, she had asked James to tear up her will, and he had done it. Would the result have been different? Explain.
-
The (identical) citizens of Boomtown have $2 million to spend either on park maintenance or private goods. Each unit of park maintenance costs $10,000. a. Graph Boomtowns budget constraint. b....
-
Because of a salary cap, National Basketball Association teams are not allowed to exceed a certain annual limit in total player salaries. Suppose the Toronto Raptors had scheduled salaries exactly...
-
In November 2010, DayTime Publishing Companys costs and quantities of paper consumed in manufacturing its 2011 Executive Planner and Calendar were as follows: Actual unit purchase price...
-
INVENTORY VALUATION METHODS: FIFO Purchases Sales: 520 kg @ 240/kg Goods Available Cost of Goods Sold 100 kg @ 110/kg 200 kg @ 100/kg 300 kg @ 90/kg 600 kg @ 58,000 total Total = 600 kg @ 58,000 100...
-
For the plane trusses supported by the spring at node 1 in Figure P3-33 (a) and (b), determine the nodal displacements and the stresses in each element. Let E = 210 GPa and A 5.0 10-4 m2 for both...
-
Consider the following reaction at a certain temperature: An equilibrium mixture contains 1.0 mole of Fe, 1.0 10 -3 mole of O 2 , and 2.0 moles of Fe 2 O 3 all in a 2.0-L container. Calculate the...
-
For a typical equilibrium problem, the value of K and the initial reaction conditions are given for a specific reaction, and you are asked to calculate the equilibrium concentrations. Many of these...
-
Describe the quality issues related to reporting revenue. What is the importance of understanding various inventory valuation methods in determining the quality of reported profits?
-
For items 20 to 21 The information below pertains to BDO Co. for 2022. Net Profit for the year 7% convertible bonds issued at par (P1,000 per bond). Each bond is convertible into 30 ordinary shares...
-
Answer the following questions about binary search trees (BSTs). (a) Draw the final BST after inserting elements 29, 38, 13, 6, 21, 17, 25, 30, 10, 8, 32 in that order. (b) Draw the tree after...
-
Find all relative extrema of the function. (If an answer does not exist, enter DNE.) f(x)=x-12x3 relative maximum (x, y) = (DNE relative minimum (x, y) =
-
In the figure below, q = 92 = -27.0 C, and the charges are separated by d = 10.0 cm. If q is located at x = 0.00 cm, find the electric potential at the midpoint between the two charges (x = 5.00 cm)....
-
Use the method of cylindrical shells to find the volume of the solid obtained by rotating the region bounded by the curves x = 1 + y, x = 0, y = 1, and y = 2 about the x-axis. Volume =
-
For the 33 nations in the Internet Use data file on the text CD, consider the following correlations: a. Which pair of variables exhibits the strongest linear relationship? b. Which pair of variables...
-
What did Lennox gain by integrating their WMS, TMS, and labor management systems?
-
Assign a name for each of the following compounds: (a) (b) (c) (d) (e) (f) `NH2 NH2
-
Draw all constitutional isomers with molecular formula C 4 H 11 N, and provide a name for each isomer.
-
Draw all tertiary amines with molecular formula C 5 H 13 N, and provide a name for each isomer. Are any of these compounds chiral?
-
Algunas acciones a desarrollar en las entregas certificadas son: Puede seleccionar ms de una opcin. Grupo de opciones de respuesta Reducir manipulacin excesiva Despacho Transporte Comunicaciones de...
-
PRACTICE EXERCISE 161. Record the following payment transactions for Letzelter Co. of Saint John,NB, during the month of November. Set up a Cash Payments Journal(page CP6) with these headings: Date,...
-
The CECL model:Multiple ChoiceConsiders historical experience but not forecasts of the future.Recognizes bad debts when it is probable that an economic sacrifice has occurred.Allows a company to use...

Study smarter with the SolutionInn App


