Naturally occurring uranium consists of two isotopes, whose masses and abundances are shown below. Only 235 U
Question:
Naturally occurring uranium consists of two isotopes, whose masses and abundances are shown below.
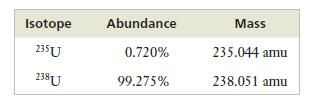
Only 235U can be used as fuel in a nuclear reactor, so uranium for use in the nuclear industry must be enriched in this isotope. If a sample of enriched uranium has an atomic weight of 235.684 amu, what percentage of 235U is present?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





