Which of these statements about how the percent ionization of a weak acid depends on acid concentration
Question:
Which of these statements about how the percent ionization of a weak acid depends on acid concentration is true?
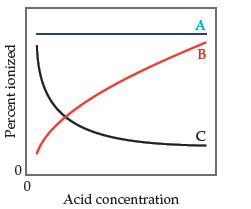
(a) Line A is most accurate because Ka does not depend on concentration.
(b) Line A is the most accurate because the percent ionization of the acid does not depend on concentration.
(c) Line B is the most accurate because as the acid concentration increases, a greater proportion of it is ionized.
(d) Line B is the most accurate because as the acid concentration increases, Ka increases.
(e) Line C is the most accurate because as the acid concentration increases, a lesser proportion of it ionized.
(f) Line C is the most accurate because as the acid concentration increases, Ka decreases.
Step by Step Answer:

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus




