Answer the following questions for this E1 reaction: (a) What is the rate expression for the reaction?
Question:
Answer the following questions for this E1 reaction:
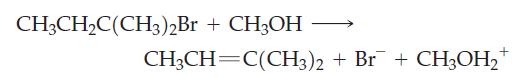
(a) What is the rate expression for the reaction?
(b) Draw the reaction profile for the reaction. Label all parts. Assume that the products are lower in energy than the reactants.
(c) What is the effect on the rate of the reaction of doubling the concentration of CH3CH2C(CH3)2Br?
(d) What is the effect on the rate of the reaction of doubling the concentration of CH3OH?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





