At 298 K, 1.00 mol BrCl(g) is introduced into a 10.0 L vessel, and equilibrium is established
Question:
At 298 K, 1.00 mol BrCl(g) is introduced into a 10.0 L vessel, and equilibrium is established in the reaction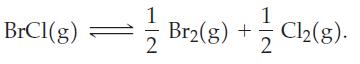 Calculate the amounts of each of the three gases present when equilibrium is established. First, use data from Appendix D, as necessary, to calculate the equilibrium constant K for the reaction. Then, relate the equilibrium amounts of the gases to their initial amounts through the extent of reaction, ξ. You might find it helpful to use a tabular approach. Finally, use the equilibrium condition Q = K to solve for ξ.
Calculate the amounts of each of the three gases present when equilibrium is established. First, use data from Appendix D, as necessary, to calculate the equilibrium constant K for the reaction. Then, relate the equilibrium amounts of the gases to their initial amounts through the extent of reaction, ξ. You might find it helpful to use a tabular approach. Finally, use the equilibrium condition Q = K to solve for ξ.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





