The dissolution of MgCO 3 (s) in NH 4 + (aq) can be represented as Calculate the
Question:
The dissolution of MgCO3(s) in NH4+(aq) can be represented as
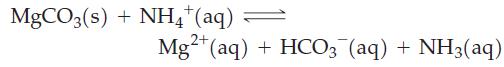
Calculate the molar solubility of MgCO3 in each of the following solutions:
(a) 1.00 M NH4Cl(aq);
(b) A buffer that is 1.00 M NH3 and 1.00 M NH4Cl;
(c) A buffer that is 0.100 M NH3 and 1.00 M NH4Cl.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





