Under what temperature conditions would the following reactions occur spontaneously? (a) 2 NH4NO3(s) - (b) I2(g) 2
Question:
Under what temperature conditions would the following reactions occur spontaneously?
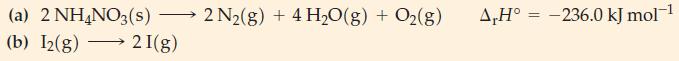
Transcribed Image Text:
(a) 2 NH4NO3(s) - (b) I2(g) 2 I(g) 2 N₂(g) + 4 H₂O(g) + O2(g) A,H° -236.0 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Analyze a The reaction is exothermic and in Example 132a we concluded that S 0 because large quantit...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Which of the four cases in Table 13.3 would apply to each of the following reactions? (B) Under what temperature conditions would the following reactions occur spontaneously? (a) The...
-
Which of the following reactions occur spontaneously as written, with the production of a measurable electric current? a. I 2 + NaBr Br 2 + 2NaI b. Li + NaCl LiCl + Na c. Li + + Na + NaLi d. Ag +...
-
Using data from Appendix 4, calculate ÎHo, ÎSo, and DGo for the following reactions that produce acetic acid: Which reaction would you choose as a commercial method for producing acetic...
-
As mentioned in Section 5.6, Sainte-Venants principle will allow particular boundary conditions to be replaced by their statically equivalent resultant. For problems (b), (c), (d),and (f) in Exercise...
-
Edington Electronics Inc. produces and sells two models of pocket calculators, XQ-103 and XQ-104. The calculators sell for $15 and $25, respectively. Because of the intense competition Edington...
-
An unstretchable steel string is used to replace a broken violin string. A length of 5.00 m of this string has a mass of 25.0 g. When in place, the new string will be 30.0 cm long and oscillate at...
-
Frontier Company, a successful efforts company, owns a working interest in an oil field in Oklahoma. The field has been producing for a number of years and is expected to be producing for another 10...
-
Presented here are long-term liability items for Borders Inc. at December 31, 2012. Prepare the long-term liabilities section of the balance sheet for Borders Inc. Bonds payable (due 2016)...
-
omplete the following table with EXACT answers: CAUTION: Watch your signs! -716 K3 3 4 2 sin/ COST tan/ CSC1 2 3 2 3 -1 (lea)
-
Arrange the entropy changes of the following processes, all at 25 C, in the expected order of increasing S, and explain your reasoning: (a) HO(1, 1 bar) (b) CO (s, 1 bar) (c) HO(1, 1 bar) 111 HO(g, 1...
-
Indicate whether each of the following changes represents an increase or a decrease in entropy in a system, and explain your reasoning: (a) The freezing of ethanol; (b) The sublimation of dry ice;...
-
How are noncash assets misappropriated?
-
Capstone Exercise One of the best ways to illustrate the value of good market research and the importance of applying the results of market research to your marketing strategy is to look at a...
-
2. Differentiate between Asset and Services Ijarah. What are the rules related to Asset Ijarah contract? 3. If you want to form a partnership relationship with your investors, what are the possible...
-
The standard deviation on your last test was 10. The average was a 70. The distribution had a normal shape (symmetrical with only one hump). Half of the people scored 70 or below. What percentage of...
-
Former US President Trump is starting to draft an economic plan for his 2024 presidential campaign. He wants to keep the tax cuts implemented by the Trump administration in 2017, the Tax Cuts and...
-
Discuss the differences between discrete probabilities and continuous probabilities. Provide three examples for each.
-
A proton is fired into a uniform electric field, opposite to the direction of the field. The protons speed upon entering the field is 3.15 x 105 m/s, and it comes to rest 5.25 cm after entering the...
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
Backflush, two trigger points, completion of production and sale (continuation of 20-33). Assume the same facts as in Problem 20-33 except now there are only two trigger points: the completion of...
-
Lean Accounting. Flexible Security Devices (FSD) has introduced a just-in-time production process and is considering the adoption of lean accounting principles to support its new production...
-
Backflushing. The following conversation occurred between Brian Richardson, plant manager at Glendale Engineering, and Charles Cheng, plant controller. Glendale manufactures automotive component...
-
Sedgwick Inc. is considering Plan 1 which is estimated to have sales of $40,000 and costs of $15,500. The company currently has sales of $37,000 and costs of $14,000. Compare plans using incremental...
-
A nonresident alien is never allowed to take the martial deduction for gifts made to his/her spouse True or False
-
please help me with the website below to answer the questions wcms_760306.pdf (ilo.org) file:///C:/Users/carin/Downloads/wcms_760306%20(1).pdf A global survey of enterprises: Managing the business...

Study smarter with the SolutionInn App


