A 0.530 kg sample of liquid water and a sample of ice are placed in a thermally
Question:
A 0.530 kg sample of liquid water and a sample of ice are placed in a thermally insulated container. The container also contains a device that transfers energy as heat from the liquid water to the ice at a constant rate P, until thermal equilibrium is reached. The temperatures T of the liquid water and the ice are given in Figure as functions of time t; the horizontal scale is set by ts = 80.0 min.(a) What is rate P?(b) What is the initial mass of the ice in the container?(c) When thermal equilibrium is reached, what is the mass of the ice produced in thisprocess?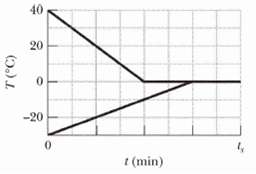
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:





