(a) From the galvanic series (Table 17.2), cite three metals or alloys that may be used to...
Question:
(a) From the galvanic series (Table 17.2), cite three metals or alloys that may be used to galvanically protect 304 stainless steel in the active state.(b) As Concept Check 17.4(b) notes, galvanic corrosion is prevented by making an electrical contact between the two metals in the couple and a third metal that is anodic to the other two. Using the galvanic series, name one metal that could be used to protect a copper??aluminum galvaniccouple.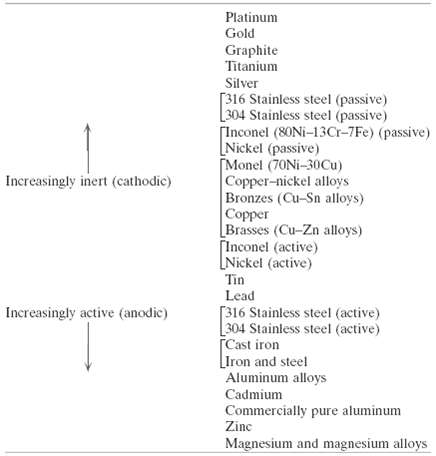
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:





