Question: A process has the transfer function of Eq. 6-14 with K = 2, ?1 = 10, ?2 = 2. If ?a has the following value:
A process has the transfer function of Eq. 6-14 with K = 2, ?1 = 10, ?2 = 2. If ?a has the following value: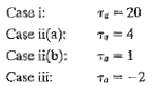 Calculate the responses for a step input of magnitude 0.5 and plot them in a single figure. What conclusions can you make concerning the effect of the zero location? Is the location of the pole corresponding to r important so long as ?1 > ?2?
Calculate the responses for a step input of magnitude 0.5 and plot them in a single figure. What conclusions can you make concerning the effect of the zero location? Is the location of the pole corresponding to r important so long as ?1 > ?2?
Case i: TE 20 Case ii(a): T, =4 Case ii(b): To Case iii: Ta = -2
Step by Step Solution
3.44 Rating (183 Votes )
There are 3 Steps involved in it
Substituting the numerical values ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
38-E-C-E-P-C (87).docx
120 KBs Word File


