Question: A sample of gas expands from V1 = 1.0 m3 and P1 = 40 Pa to V2 = 4.0 m3 and P2 = 10 Pa
A sample of gas expands from V1 = 1.0 m3 and P1 = 40 Pa to V2 = 4.0 m3 and P2 = 10 Pa along path B in the p-V diagram in Figure. It is then compressed back to V1 along either path A or path C. Compute the net work done by the gas for the complete cycle along(a) Path BA and(b) PathBC.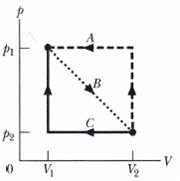
Pr P 10 V V -V
Step by Step Solution
3.31 Rating (166 Votes )
There are 3 Steps involved in it
The net work may be computed as a sum of works for the individual processes involved or as the area ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-F-L (486).docx
120 KBs Word File


