Question: A thermodynamic system is taken from state A to state B to state C., and then back to A, as shown in the p-V diagram
A thermodynamic system is taken from state A to state B to state C., and then back to A, as shown in the p-V diagram of Figure a. The vertical scale is set by ps = 40Pa, and the horizontal scale is set by Vs = 4.0 m3.(a) ?? (g) Complete the table in Figure b by inserting a plus sign, a minus sign, or a zero in each indicated cell.(h) What is the net work done by the system as it moves once through the cycleABCA?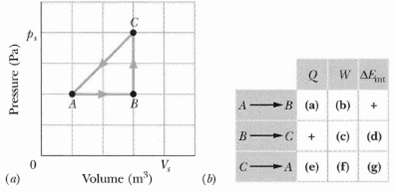
W AE A B (a) (b) (c) (d) V, Volume (m) A (e) (f) ()(g) (a) (b) Pressure (Pa)
Step by Step Solution
3.39 Rating (180 Votes )
There are 3 Steps involved in it
During process A B the system is expanding doing work on its environment so W 0 and since AEint 0 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-F-L (439).docx
120 KBs Word File


