As shown in Figure, water (W) and n-butanol (B) can form a three-phase system at 101 kPa.
Question:
As shown in Figure, water (W) and n-butanol (B) can form a three-phase system at 101 kPa. For a mixture of overall composition of 60 mol% W and 40 mol% B, use a simulation computer program and the UNIFAC method to estimate:(a) Dew-point temperature and composition of the first drop of liquid.(b) Bubble-point temperature and composition of the first bubble of vapor.(c) Compositions and relative amounts of all three phases for 50 mol%vaporization,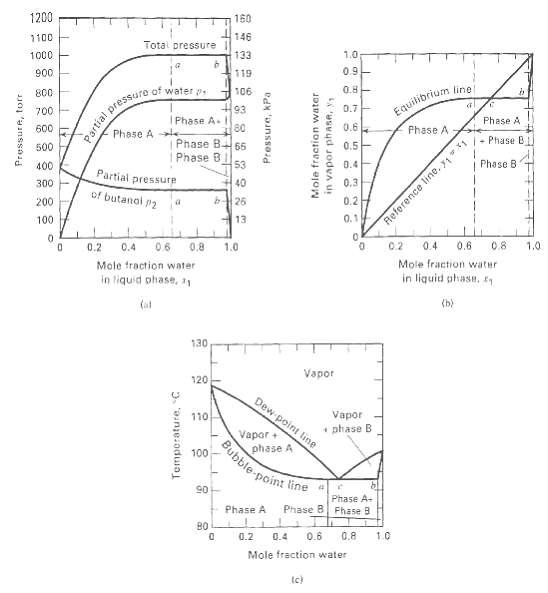
Transcribed Image Text:
1200 160 1100 146 Total pressure 1000 133 1.0 0.9 900 119 0.8- Partial press. Phase A le 106 800 of water p. Equilibrium ling 93 I Phase A. 80 0.7 700 Phase A 0.6 600 Phase A Phase B Phase B65 0.5- 500 1 Phase B 53 Phase B 400 0.4 Partial pressure 40 0.3 300 of butanol p2 200 0.2 13 0.1 100 06 0.6 0.8 1.0 1.0 0.2 0.4 0.2 0.4 0.8 Mole fraction water in liquid phase, 1 Mole fraction water in liquid phase, 11 (b) (al 130 Vapor 120 Dew point line Vapor 110 * phase B Vapor + phase A Bubble noint line 100 90 Phase A- Phase B Fhase 9 Phase A B0 0.6 0.8 1.0 0.2 0.4 Mole fraction water Pressure, tor Temperature, C Pressure, kPa Mole fraction water in vapor phaSA, y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
Use the CHEMCAD simulator with UNIFAC LLE for Kvalues with the threephase flash model The following ...View the full answer

Answered By

HILLARY KIYAYI
I am a multi-skilled, reliable & talented Market analysis & Research Writer with a proven ability to produce Scholarly Papers, Reports, Research and Article Writing and much more. My ultimate quality is my English writing/verbal skill. That skill has proven to be the most valuable asset for project writing, Academic & Research writing, Proofreading, HR Management Writing, business, sales, and a variety of other opportunities.
4.80+
24+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A system has a block diagram as shown in Figure AP2.2. Determine the transfer function T(s) = Y2(s) / R1(s) It is desired to decouple Y2(s) from R1(s) by obtaining T(s) = 0. Select G5(s) in terms of...
-
A system has a block diagram as shown in Figure P3.26. Determine a state variable model and the state transition matrix Φ(s). R(s) Msi 25 FIGURE P3.26 Feedback system
-
A weather glass, as shown in FIGURE 15-45, is used to give an indication of a change in the weather. Does the water level in the neck of the weather glass move up or move down when a low-pressure...
-
Compare the ACA passed in 2010 with the Massachusetts health plan adopted in 2006 (use the Internet to obtain more details than we have provided in this chapter). Be sure to note the similarities and...
-
At July 31, Southco Limited had an unadjusted cash balance of $16,320. An examination of the July bank statement shows a balance of $15,840 on July 31; outstanding cheques $2,300; deposits in transit...
-
When attenuation occurs in an analog signal, what hardware device is used to restore the original signal? When attenuation occurs in a digital signal, what hardware device is used to restore the...
-
The ANES asks people about their smoking behavior. I took these variables and created a dichotomous variable: 0 = dont currently smoke, 1 = currently smoke. Use this as your dependent variable and...
-
Solve the following linear programming model graphically: maximize Z = 3x1 + 2x2 subject to 2x1 + 4x2 22 -x1 + 4x2 10 4x1 - 2x2 14 x1 - 3x2 1 x1, x2 0
-
Kubin Company's relevant range of production is 18,000 to 22.000 units. When it produces and sells 20,000 units, its average costs per unit are as follows: Direct materials Direct labor Variable...
-
Erica and Bob participate in a friendly Hackathon that allows each to solve one question a day out of the three offered. There will be one easy, one medium and one hard question, with points awarded...
-
A liquid containing 30 mol% toluene, 40 mol% ethylbenzene, and 30 mol% water is subjected to a continuous, flash distillation at a total pressure of 0.5 atm. Assuming that mixtures of ethylbenzene...
-
Repeat Example 4.19 for a temperature of 25oC. Are the changes significant?
-
You Decide: Should start-up costs be capitalized or expensed? Your spouse is setting up a home-based Web design business. Her purpose for setting up the business is to earn some extra income now and,...
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
How do individual differences in cognitive styles, such as analytical versus intuitive thinking, impact problem-solving approaches and decision-making processes within teams ?
-
In Russian government, do you think that Russian Military Performance is good in warfare against Ukraine? Explain.
-
Why do you think the competing values framework is important to an organization's effectiveness? Describe the four profiles of the competing values framework. Identify one of the profiles and provide...
-
What two essential elements are necessary for achieving meaningful translated reporting results?
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
Discuss the often deep-rooted barriers that prevent individuals from accessing employment and obtaining equality at work. How, in your opinion, could these barriers be overcome?
-
Can a near-perfect separation be made with gas permeation? If not, why not?
-
Repeat part (a) of Exercise 14.10 for a two-stage stripping cascade and a two-stage enriching cascade, as shown in Figure 14.14. However, select just one set of reasonable cuts for the two stages of...
-
What is the pseudo-steady-state assumption used in the shrinking-core leaching model?
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


