Question: Below is shown a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature, for the diffusion
Below is shown a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature, for the diffusion of iron in chromium. Determine values for the activation energy and preexponential.
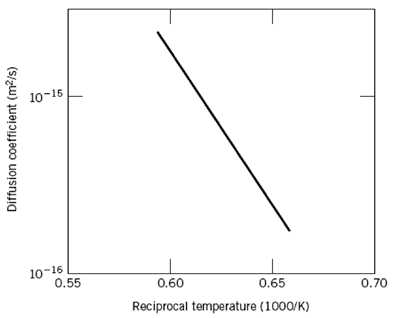
10-15 10-16 0.55 0.60 0.65 0.70 Reciprocal temperature (1000/K) Diffusion coefficient (m/s)
Step by Step Solution
3.50 Rating (173 Votes )
There are 3 Steps involved in it
This problem asks us to determine the values of Q d and D 0 for the diffusion of Fe in Cr from ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (148).docx
120 KBs Word File


