A chemical reaction with stoichiometry A ? products is said to follow an n th -order rare
Question:
A chemical reaction with stoichiometry A ? products is said to follow an nth-order rare law if A is consumed at a rate proportional to the nth power of its concentration in the reaction mixture. If rA is the rate of consumption of A per unit reactor volume, then rA [mol/ (L?s)] = kCnA?where CA?(mol/L) is the reactant concentration, and the constant of proportionality k is the reaction rate constant. A reaction that follows this law is referred to as an nth order reaction. The rate constant is a strong function of temperature but is independent of the reactant concentration.
(a) Suppose a first-order reaction (n = 1) is carried out in an isothermal batch reactor of constant volume V. Write a material balance on A and integrate it to derive the expression CA = CA0 exp(?kt) where CA0 is the concentration of A in the reactor at t = 0.
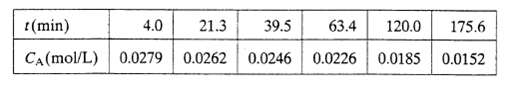
(b) The gas-phase decomposition of sulfuryl chloride SO2C12 ? SO2 + Cl2?is thought to follow a first-order rate law. The reaction is carried out in a constant-volume isothermal batch reactor and the concentration of SO2C12 is measured at several reaction times, with the following results: Verify the proposed rate law graphically [i.e.. demonstrate that the expression given in part (a) fits the data for CA(t)] and determine the rate constant k, giving both its value and its units.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





