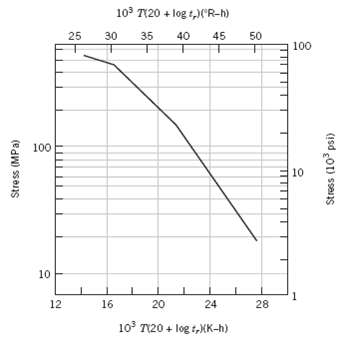
Consider an 18-8 Mo stainless steel component (Figure) that is exposed to a temperature of 500(C (773
Question:
Consider an 18-8 Mo stainless steel component (Figure) that is exposed to a temperature of 500(C (773 K). What is the maximum allowable stress level for a rupture lifetime of 5 years? 20 years?
Transcribed Image Text:
10° 7(20 + log t,)R-h) 25 30 35 40 45 50 100 100 10 10 12 16 20 24 28 10 T(20 + log t,MK-h) Stress (MPa) пт (Isd „01) ssans
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
We are asked in this problem to calculate the stress levels at w...View the full answer

Answered By

Joseph Njoroge
I am a professional tutor with more than six years of experience. I have helped thousands of students to achieve their academic goals. My primary objectives as a tutor is to ensure that students do not have problems while tackling their academic problems.
4.90+
10+ Reviews
27+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
Consider an 18-8 Mo stainless steel component (Figure 8.35) that is exposed to a temperature of 500C (773 K). What is the maximum allowable stress level for a rupture lifetime of 5 years? 20 years?
-
A 5.0-cm-diameter cylinder is heated to a temperature of 200C, and air at 30C is forced across it at a velocity of 50 m/s. If the surface emissivity is 0.7, calculate the total heat loss per unit...
-
A semi-infinite slab of copper is exposed to a constant heat flux at the surface of 0.5 MW/m2. Assume that the slab is in a vacuum, so that there is no convection at the surface. What is the surface...
-
Monroe Inc. is an all-equity firm with 500,000 shares outstanding. It has $2,000,000 of EBIT, and EBIT is expected to remain constant in the future. The company pays out all of its earnings, so...
-
1. Oxnard Industries produces a product that requires 2.6 pounds of materials per unit. The allowance for waste and spoilage per unit is .3 pounds and .1 pounds, respectively. The purchase price is...
-
Refer to the Real Estate DataSaskatoon online, which reports information on home listings. a. Compute the mean and standard deviation of the distribution of the list prices for the homes. Assume this...
-
What is viral marketing? LO.1
-
Assume you are the vice president of operations for HG Lang and you are preparing for a meeting to discuss the 2012 financial results with Adrienne Aiello, the company president. HG Lang Designs...
-
Required Information (The following information applies to the questions displayed below) The following unadjusted trial balance is prepared at fiscal year-end for Nelson Company Nelson Company uses...
-
You are the audit senior on the audit of Great Eastern Hotel (GEH). This two-star hotel is located in a major coastal city and as such is prone to seasonal fluctuations. The 200-room hotel is open...
-
For an 18-8 Mo stainless steel (Figure), predict the time to rupture for a component that is subjected to a stress of 80MPa (11,600psi) at 700?C (973 K). 10 T(20 + log t,(R-h) 25 30 35 40 45 50 100...
-
Consider the sugar?water phase diagram of Figure. (a) ? How much sugar will dissolve in 1500 g water at 90?C (194?F)? (b) If the saturated liquid solution in part (a) is cooled to 20?C (68?F), some...
-
Locate the Treasury bond in Figure 6.3 maturing in February 2037. Is this a premium or a discount bond? What is its current yield? What is its yield to maturity? What is the bid-ask spread for a...
-
The Intel Outsourcing case case explores the make-versus-buy decision for the well-known chip maker. Use The Strategic Sourcing framework to examine this important decision for Intel. Use the...
-
Final-year students enrolled in the Interactive Multimedia course at Edith Cowan University are required to develop skills and expertise in managing the design and development of client websites. The...
-
Find the minimum tractive effort required for vehicle to maintain 70mph speed at 5%upgrade through an air density of 0.002045 slug/ft^3. Show all steps and unit conversion please Problem 2:...
-
Sanburn writes about the conflict of decreasing funding and enrollment for community colleges and the increasing value of an associate degree. Explain how those two factors can co - exist at the same...
-
Rare beauty new Shampoo and Conditioner Branding Strategy What is the branding strategy for your organization? What is the purpose of your brand? How will you differentiate yourself from domestic...
-
Is it possible for an employee to have personal values that are inconsistent with the values of the organization? If so, how is this inconsistency likely to affect the employees behaviour and...
-
Horse serum containing specific antibody to snake venom has been a successful approach to treating snakebite in humans. How do you think this anti-venom could be generated? What are some advantages...
-
In recent years, the notion of a smart grid has emerged. Do a web search and research the smart grid concept. How would the smart grid differ from the traditional grid?
-
The diffusion coefficients for carbon in nickel are given at two temperatures are as follows: T (C) _________ D (m2 / s) 600 ............ 5.5 10-14 700............. 3.9 10-13 (a) Determine the...
-
The accompanying figure shows a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature for the diffusion of gold in silver. Determine values...
-
The accompanying figure shows a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature for the diffusion of vanadium in molybdenum....
-
Suppose you bought a bon with an annual coupon rate of 6.5 percent one year ago for $1,032. The bond sells for $1,020 today. a. Assuming a $1,000 face value, what was your total dollar return on this...
-
During the year 2021, William has a job as an accountant, he earns a salary of $100,000. He has done some cleaning services work on his own (self-employed), where he earned a net income of $50,000....
-
Fixed cost per unit is $7 when 25,000 units are produced and $5 when 35,000 units are produced. What is the total fixed cost when 30,000 units are produced? Group of answer choices $150,000....
Statistics For Six Sigma Green Belts With Minitab And JMP 1st Edition - ISBN: 013701712X - Free Book

Study smarter with the SolutionInn App


