Consider the velocity graph in Figure. Assuming x = 0 at t = 0, write correct algebraic
Question:
Consider the velocity graph in Figure. Assuming x = 0 at t = 0, write correct algebraic expressions for x(t), v(t), and a(t) with appropriate numerical values inserted for allconstants.
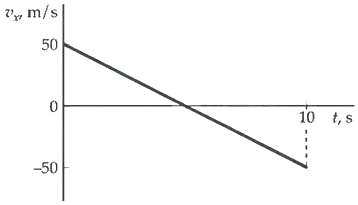
Transcribed Image Text:
V, m/s 50 10 t, s -50
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
vt note that a i...View the full answer

Answered By

Brown Arianne
Detail-oriented professional tutor with a solid 10 years of experience instilling confidence in high school and college students. Dedicated to empowering all students with constructive feedback and practical test-taking strategies. Effective educator and team player whether working in a school, university, or private provider setting. Active listener committed to helping students overcome academic challenges to reach personal goals.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Mechanics questions
-
Figure EX26.9 shows a graph of V versus x in a region of space. The potential is independent of y and z. What is E x at (a) X = -2 cm, (b) X = 0 cm, (c) X = 2 cm? V (V) 10- x (m) -32 -1 1 2 3 107...
-
FIGURE EX17.1 is a snapshot graph at t = 0 s of two waves approaching each other at 1.0 m/s. Draw six snapshot graphs, stacked vertically, showing the string at 1 s intervals from t = 1 s to t = 6 s....
-
Write the equilibrium-constant expressions and obtain numerical values for each constant in (a) The basic dissociation of aniline, C6H5NH2. (b) The acidic dissociation of methyl ammonium...
-
Nisha has completed her MBA and has joined a company which was going to raise fund from long term sources such as Debt and Equity. Nisha was asked by her manager to prepare a report on which could be...
-
Is gravitational force acting on a person who falls off a cliff? On an astronaut inside an orbiting space shuttle?
-
Is the equation t 2 y'(t) = (t + 4)/y 2 separable?
-
A hotel front-desk agents job description contains the following task: Greet arriving guests in a friendly, courteous, and efficient manner. Rewrite this task so that it contains objective and...
-
On January 1, 2010, Travers Company acquired 90 percent of Yarrow Companys outstanding stock for $720,000. The 10 percent noncontrolling interest had an assessed fair value of $80,000 on that date....
-
CAN SOMEONE PLEASE HELP ME Answer to Question c Statement of Financial Position as at 31 December 2019
-
For next month, a hotel manager forecasts revenue of $800,000. 60% of the revenue to be generated in the month will be made to customers who will not pay their bills within the same month as they are...
-
The one-dimensional motion of a particle is plotted in Figure. (a) What is the acceleration in the intervals AB, BC, and CE? (b) How far is the particle from its starting point after 10 s? (c) Sketch...
-
Starting at one station, a subway train accelerates from rest at a constant rate of 1.0 m/s 2 for half the distance to the next station, then slows down at the same rate for the second half of the...
-
The region near the South Pole in Antarctica has an altitude of about 3000 m, making the air pressure lower than at sea level. Explain why it is more difficult for an airplane to take off from the...
-
In the circuit of Fig. 4-51 write two loop equations using I 1 and I 2 . Then find the currents and node voltages. A 3A ( 4 3 V 792 B +1 D w 392 12 C
-
The capacitor in the circuit shown in Fig. 7-37 has initial charge Q 0 = 800 C, with polarity as indicated. If the switch is closed at t = 0, obtain the current and charge, for t > 0. 100 V (+ 10 4 F
-
A gift shop sells 400 boxes of scented candles a year. The ordering cost is \($60\) for scented candles, and holding cost is \($24\) per box per year. What is the economic order size for scented...
-
Kay Vickery is angry with Gene Libby. He is behind schedule developing supporting material for tomorrows capital budget committee meeting. When she approached him about his apparent lackadaisical...
-
Tharpe Painting Company is considering whether to purchase a new spray paint machine that costs \($3,000\) . The machine is expected to save labor, increasing net income by \($450\) per year. The...
-
E25-11 Baxter Fabricators, Inc.. completed two jobs in June 20X4. Baxter recorded the fol- lowing costs assigned to the jobs by the company's activity-based costing system: Allocated Cost Activity...
-
suppose a nickel-contaminated soil 15 cm deep contained 800 mg/kg Ni, Vegetation was planted to remove the nickel by phytoremediation. The above-ground plant parts average 1% Ni on a dry-weight bas...
-
How do you use the HendersonHasselbalch equation to calculate the pH of a buffer containing a base and its conjugate acid? Specifically, how do you determine the correct value for pK a ?
-
A vertical cylinder with a movable piston contains 1.00 mol of a diatomic ideal gas. The volume of the gas is Vi, and its temperature is Ti. Then the cylinder is set on a stove and additional weights...
-
A container has a mixture of two gases: n1 mol of gas 1 having molar specific heat C1 and n2 mol of gas 2 of molar specific heat C2. (a) Find the molar specific heat of the mixture. (b) What If? What...
-
During the compression stroke of a certain gasoline engine, the pressure increases from 1.00 atm to 20.0 atm. If the process is adiabatic and the fuelair mixture behaves as a diatomic ideal gas, (a)...
-
How does budgeting household expenses differ from budgeting business expenses? What are the similarities?
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....

Study smarter with the SolutionInn App


