A container holds a mixture of three non reacting gases: 2.40 mol of gas 1 with Cv1
Question:
A container holds a mixture of three non reacting gases: 2.40 mol of gas 1 with Cv1 = 12.0 J/mol ?? K, 1.50 mol of gas 2 with Cv2 = 12.8 J/mol ?? K and 3.20 mol of gas 3 with Cv3 = 20.0 J/mol ?? K. What is CV of the mixture?
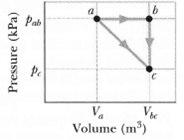
Transcribed Image Text:
Pab Ve V. Volume (m) Pressure (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
When the temperature changes by AT the internal energ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:
Students also viewed these Thermodynamics questions
-
A gas mixture of 1 kmol carbon monoxide, 1 k mol nitrogen, and 1 k mol oxygen at 25C, 150 kPa, is heated in a constant pressure SSSF process. The exit mixture can be assumed to be in chemical...
-
A container has a mixture of two gases: n1 mol of gas 1 having molar specific heat C1 and n2 mol of gas 2 of molar specific heat C2. (a) Find the molar specific heat of the mixture. (b) What If? What...
-
A gas mixture of 1 pound mol carbon monoxide, 1 pound mol nitrogen, and 1 pound mol oxygen at 77 F, 20 lbf/in 2, is heated in a constant pressure SSSF process. The exit mixture can be assumed to be...
-
How would this photo be different if the two people were both Americans?
-
Apply the imputed interest rules in the following situations. a. Mike loaned his sister Shonda $90,000 to buy a new home. Mike did not charge interest on the loan. The Federal rate was 4%. Shonda...
-
In Figure 11-2, if planned saving was less than planned investment, what would be true of the interest rate in relation to its equilibrium value? How would the interest rate adjust?
-
Explain how conflict can be either functional or dysfunctional, and distinguish among various types of conflict.p. 408
-
Cybernetics Inc. issued $60 million of 5% three-year bonds, with coupon paid at the end of every year. The effective interest rate at the beginning of Years 1, 2, and 3 was 8%, 5%, and 2%. Required:...
-
P16-1B Cheese Farms is a grower of hybrid seed corn for Steenbergen Genetics Corporation. It has had two exceptionally good years and has elected to invest its excess funds in bonds. The selected...
-
The Bayside Art Gallery is considering installing a video camera security system to reduce its insurance premiums. A diagram of the eight display rooms that Bayside uses for exhibitions is shown in...
-
Under constant pressure, the temperature of 2.00 mol of an ideal monatomic gas is raised 15.0 K. What are? (a) The work V/done by the gas, (b) The energy transferred as heat Q, (c) The change Eint,...
-
One mole of an ideal diatomic gas goes from a to c along the diagonal path in Figure. The scale of the vertical axis is set by pab = 5.0kPa and pc = 2.0 kPa, and the scale of the horizontal axis is...
-
Explain why T 1 T 2 .
-
Idenfity whether the following book - tax adjustments are permanent or temporary differences. ( a ) Federal Income Tax Expense ( b ) Depreciation Expense ( c ) Accrued Compensation ( d ) Dividends...
-
2 . ) Pozycki, LLC has reported losses of $ 1 0 0 , 0 0 0 per year since its founding in 2 0 1 6 . For 2 0 2 3 , Pozycki anticipates a profit of about $ 1 0 0 , 0 0 0 . There are 3 equal members of...
-
Elena is a single taxpayer for tax year 2023. On April 1st, 2022, Elena's husband Nathan died. On July 13, 2023, Elena sold the residence that Elena and Nathan had each owed and used as their...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $56,600 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
Problem 3: A large rectangular plate is loaded in such a way as to generate the unperturbed (i.e. far-field) stress field xx = Cy; yy = -C x; Oxy = 0 The plate contains a small traction-free circular...
-
Create customer segments in a marketing campaign.
-
An item of depreciable machinery was acquired on 1 July 2009 for $120,000 by cash It is expected to have a useful life of 10 years and zero salvage value On 1 July 2012, it was decided to revalue the...
-
Define net investment, replacement investment, new investment and gross investment.
-
Stay Dry! You tie a cord to a pail of water, and you swing the pail in a vertical circle of radius 0.600 m. What minimum speed must you give the pail at the highest point of the circle if no water is...
-
A bowling ball weighing 71.2 N (16.0 Ib) is attached to the ceiling by a 3.80-m rope. The ball is pulled to one side and released; it then swings back and forth as a pendulum. As the rope swings...
-
Two ropes are connected to a steel cable that supports a hanging weight as shown in Fig. 5.59. (a) Draw a free-body diagram showing all of the forces acting at the knot that connects the two ropes to...
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....
-
What is the definition of substantially appreciated inventory? A. Inventory with a FMV greater than its basis B. Inventory and unrealized receivables with a FMV greater than their basis C. Inventory...

Study smarter with the SolutionInn App


