Develop the feasible product composition regions for the system of figure if the feed composition is 50
Question:
Develop the feasible product composition regions for the system of figure if the feed composition is 50 mol% chloroform, 25 mol% methanol, and 25 mol%acetone.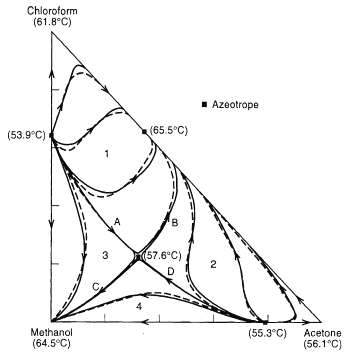
Transcribed Image Text:
Chloroform (61.8°C) 1 Azootrope (65.5°C) (53.9°C) (57.6°C) 3 D. Methanol (55.3°C) Acetone (64.5°C) (56.1°C) B.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
The feed composition is shown at F Thus the feed is in Region 1 which is bounded by distillation bou...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Chemical Engineering questions
-
Develop the feasible product-composition regions for the system of figure, using FeedF1. D2 D, (b) B, Region Region 2 (a) B3 B.
-
For the system of figure below find the transfer function G(s)=X 1 (s)/F(s). K1 = 4 N/m K2 = 5 N/m f(t) fy, = 3 N-s/m M =Ikg Jv2=3N-s/m M,= 2 kg fva = 2 N-s/m! %3D
-
For the system of Figure 1, what happens to the two cylinders in each case? a. Push-button 1-PB is momentarily depressed. b. Push-button 2-PB is momentarily depressed. Note that cylinder 2 does not...
-
Oliveras Company had net credit sales during the year of $800,000 and cost of goods sold of $500,000. The balance in accounts receivable at the beginning of the year was $100,000, and the end of the...
-
A $110,000 payment is made on a long-term liability. Of this amount, $10,000 represents interest. Which of the following is not true for the recording of this transaction? a. Reduce liabilities by...
-
Determine the NPSH available when a pump draws carbon tetrachloride at 65C (sg = 1.48) from a tank whose level is 1.2 m below the pump inlet. The energy losses in the suction line total 0.72 m and...
-
Using the data from Problem 11.2, determine which means are different using a = 0.05. AppendixLO1
-
Refer to Theory in Practice vignette 7.4, describing how The Blackstone Group proposed to account for the carried interest to be received from future earnings of unconsolidated firms it has invested...
-
Milan Statuary manufactures bust statues of famous historical figures. All statues are the same size. Each unit requires the same amount of resources. The following information is from the static...
-
1. Explain why Kroger is recognized by Forbes as one of the most generous companies in America? Is being generous consistent with (or inconsistent with) being highly profitable? Discuss. 2. Which...
-
For the same conditions as Exercise 11.5, use a process-simulation program with the UNIFAC equation to calculate a portion of a distillation curve at 1 atm.
-
Repeat Example 11.3, but with ethanol as the solvent.
-
From the following information relating to the fixed assets of a business prepare the following accounts as they should appear in the ledger: (a) plant and machinery; (b) motor vehicles; (c) plant...
-
Find f(x) if y = f(x) satisfies dy dx 60yx and the y-intercept of the curve y = f(x) is 6. f(x)
-
Data on the gasoline tax per gallon ( in cents ) as of a certain date for the 5 0 U . S . states and the District of Columbia are shown below. State Gasoline Tax per Gallon State Gasoline Tax per...
-
Write a function report card where the user can enter each of his grades, after which the program prints out a report card with GPA. Remember to ask the user how many classes he took (think - why...
-
VA= 18/ A c = ? 1. For the arrangement of cable and pulleys in the figure, the velocities and accelerations of two of the bodies involved are indicated. Determine the velocity and acceleration of the...
-
Suppose there are two electric charges in 2D planeR2; one is on y-axis and the other is on x-axis: 45km 0 p1:=0,p2:=30km.(1) Thechargesareq1=4.5Catp1andq2=3.0Catp2. (a) Determine the electric field...
-
Dilution What is dilution, and why does it occur when warrants are exercised?
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
List three factors that can cause a financial crisis.
-
Fifty thousand lb/h of a 20 wt% aqueous solution of NaOH at 120F is to be fed to an evaporator operating at 3.7 psia, where the solution is concentrated to 40 wt% NaOH. The heating medium is...
-
A desublimation unit of the heat-exchanger type is to be sized for the recovery of 200 kg/h of benzoic acid (BA) from a gas stream containing 0.8 mol% BA and 99.2 mol% N 2 . The gas enters the unit...
-
A bar of 98 wt% Al with 2 wt% of Fe impurity is subjected to one pass of zone refining. The solidliquid distribution coefficient for the impurity is 0.29. If z/l = 10 and the resulting bar is cut off...
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...

Study smarter with the SolutionInn App


