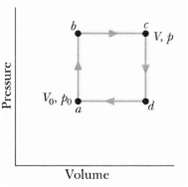
Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Assume
Question:
Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Assume that p = 2p0, V = 2V0, p0 = 1.01 x 105 Pa, and V0 = 0.0225 m3. Calculate
(a) The work done during the cycle,
(b) The energy added as heat during stroke abc and
(c) The efficiency of the cycle.
(d) What is the efficiency of a Carnot engine operating between the highest and lowest temperatures that occur in the cycle?
(e) Is this greater than or less than the efficiency calculated in(c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:





