Question: Ion exclusion is a process that uses ion-exchange resins to separate nonionic organic compounds from ionic species contained in a polar solvent, usually water. The
Ion exclusion is a process that uses ion-exchange resins to separate nonionic organic compounds from ionic species contained in a polar solvent, usually water. The resin is pre-saturated with the same ions as in the solution, thus eliminating ion exchange. However, in the presence of the polar solvent, resins undergo considerable swelling by absorbing the solvent. Experiments have shown that a nonionic solute will distribute between the solution outside the resin and the solution within the resin, while the ions can only exchange. A feed solution of 1,000 kg contains 6 wt% NaCl, 35 wt% glycerol, and 47 wt% water. This solution is to be treated with Dowex-50 ion-exchange resin in the sodium form, after prewetting with water, to recover 75% of the glycerol. The following data for the glycerol distribution coefficient,![]() were reported by Asher and Simpson [J. Phys. Chem., 60,518-521 (1956)]:
were reported by Asher and Simpson [J. Phys. Chem., 60,518-521 (1956)]: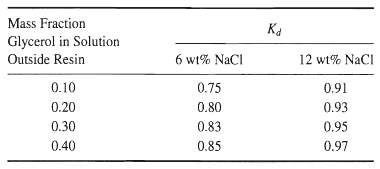 If the prewetted resin contains 40 wt% water, determine the kilograms of resin (dry basis)required.
If the prewetted resin contains 40 wt% water, determine the kilograms of resin (dry basis)required.
mass fraction in solution inside resin Ka mass fraction in solution outside resin
Step by Step Solution
3.47 Rating (163 Votes )
There are 3 Steps involved in it
Let R kg of dry resin needed Then the water in the prewetted resin 4060 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (528).docx
120 KBs Word File


