Knowing that the assembly of Prob. 18.65 is initially at rest (w = 0) when a couple
Question:
Knowing that the assembly of Prob. 18.65 is initially at rest (w = 0) when a couple of moment M0 = (18lb ?? ft)i is applied to the shaft, determine
(a) The resulting angular acceleration of the assembly,
(b) The dynamic reactions at points A and B immediately after the couple has been applied.
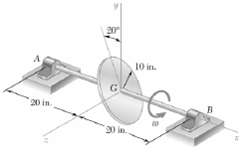
Transcribed Image Text:
901 10 in.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (18 reviews)
a 0 x B Use principal axes x y z as shown co0 a a cosei asine j Moments of ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Vector Mechanics for Engineers Statics and Dynamics
ISBN: 978-0073212227
8th Edition
Authors: Ferdinand Beer, E. Russell Johnston, Jr., Elliot Eisenberg, William Clausen, David Mazurek, Phillip Cornwell
Question Posted:
Students also viewed these Mechanical Engineering questions
-
Knowing that the assembly of Prob. 18.68 is initially at rest ( = 0) when a couple of moment M0 = (36 N ?? m)j is applied to the shaft, determine (a) The resulting angular acceleration of the...
-
The assembly of Prob. 18.63 is initially at rest ( = 0) when a couple M0 is applied to axle AB. Knowing that the resulting angular acceleration of the assembly is = (150 rad/s2)i, determine (a) The...
-
A 0.42 kg shuffleboard disk is initially at rest when a player uses a cue to increase its speed to 4.2 m/s at constant acceleration. The acceleration takes place over a 2.0 m distance, at the end of...
-
1. Suppose India follows China and accelerates its industrialization. For India, this will: A. Decrease imports and raise net exports B. Increase imports and lower net exports C. Increase imports and...
-
Mel Morgan works as a cook for an Applebee's diner. His regular pay rate is $10 per hour, with time-and-a-half for hours in excess of 40 per week. Morgan's payroll deductions include withheld income...
-
Use the same directions for Problems as for Problems 1. Describe in each case what differences are caused by the equations being non autonomous. a. y' = y(y -t) b. y' = (y - 1)2
-
Summarize the computation of cost of goods manufac- tured Use any dollar amounts. 3
-
During a meeting of the management committee of Edsel Corporation, a number of proposals are made to alleviate its weak cash position and improve income. Evaluate and comment on both the immediate...
-
Year Year 1 Year 2 Total Rooms available 1 8 , 2 5 0 1 8 , 2 5 0 Rooms occupied 1 0 , 9 5 0 1 2 , 7 7 5 Occupancy Levels 6 0 % 7 0 % Room Rate 3 5 0 4 0 0 Rev Par 2 1 0 2 8 0 Total Revenues 5 , 9 8 2...
-
Lobers, Inc., has two investment proposals, which have the following characteristics: For each project, compute its payback period, its net present value , and its profitability index using a...
-
The sheet-metal component shown is of uniform thickness and has a mass of 600 g. It is attached to a light axle supported by bearings at A and B located 150 mm apart. The component is at rest when it...
-
Knowing that the shaft of Prob. 18.66 is initially at rest (w = 0), determine (a) The Magnitude of the couple M0 = M0i required to impart to the shaft an angular acceleration a = (150 rad/s2)i, (b)...
-
Compute the results in Problems 3136. Leave your answers in scientific notation. a. (6 x 107) (4.8 x 10-6) 2.4 x 105
-
Silver Company makes a product that is very popular as a Mothers Day gift. Thus, peak sales occur in May of each year, as shown in the companys sales budget for the second quarter given below: April...
-
Among the following statements, select the ones which have a positive environmental impact. Choose several answers Minimising the impact of a product on the environment Avoiding the destruction of a...
-
Developing Financial Statements: All organizations, including those in the healthcare industry, need to make money to be profitable and survive. Financial statements, such as balance sheets, profit...
-
The engineers estimated that on average, fuel costs, assuming existing routes and number of flights stay the same, would decrease by almost 18% from an average of 42,000 gallons of jet fuel per...
-
It's the latest Berkeley trend: raising chickens in a backyard co-op coop. (The chickens cluck with delight at that joke.) It turns out that Berkeley chickens have an unusual property: their weight...
-
A nine-month T-bill with a face value of $10,000 currently sells for $9,600. Calculate the annualized simple interest rate. AppendixLO1
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
What is the net ionic equation for the reaction that occurs when an aqueous solution of KI is added to an aqueous solution of Pb(NO 3 ) 2 ?
-
A gas mixture at 600 R and 20 psia consists of 1 lbm of CO2 and 3 lbm of CH4. Determine the partial pressure of each gas and the apparent molar mass of the gas mixture.
-
A 0.3-m3 rigid tank contains 0.6 kg of N2 and 0.4 kg of O2 at 300 K. Determine the partial pressure of each gas and the total pressure of the mixture.
-
A gas mixture at 350 K and 300 kPa has the following volumetric analysis: 65 percent N2, 20 percent O2, and 15 percent CO2. Determine the mass fraction and partial pressure of each gas.
-
How does the life time analysis differ from the basic customer profitability approach(500 words)
-
The security analysis research reports, published by sell side analysts working for security firms and FINRA, purport to sell an analyst's investment ideas to an investor in exchange for...
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....

Study smarter with the SolutionInn App


