Question: A liquid-phase chemical reaction A ? B takes place in a well-stirred tank. The concentration of A in the feed is C A0 (mol/m 3
A liquid-phase chemical reaction A ? B takes place in a well-stirred tank. The concentration of A in the feed is CA0 (mol/m3), and that in the tank and outlet stream is CA (mol/m3). Neither concentration varies with time. The volume of the tank contents is V(m3) and the volumetric flow rate of the inlet and outlet streams is v (m3/s). The reaction rate (the rate at which A is consumed by reaction in the tank) is given by the expression r (mol A consumed/s) = kVCA where k is a constant.
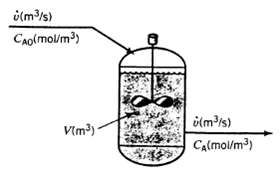
(a) Is this process continuous, batch, or semi batch? Is it transient or steady-state?
(b) What would you expect the reactant concentration CA to equal if k = 0 (no reaction)? What should it approach if k ? ? (infinitely rapid reaction)?
(c) Write a differential balance on A, stating which terms in the general balance equation (accumulation = input + generation ? output ? consumption) you discarded and why you discarded them. Use the balance to derive the following relation between the inlet and outlet reactant concentrations: CA = CA0/1 + kV/v Verity that this relation predicts the results in part (b).
(m/s) Cao(mol/m3) V(m) (m/s) CA(mol/m)
Step by Step Solution
3.34 Rating (166 Votes )
There are 3 Steps involved in it
a Continuous Steady State b C ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (101).pdf
180 KBs PDF File
13-E-C-E-C-P (101).docx
120 KBs Word File


