Magnesium oxide has the rock salt crystal structure and a density of 3.58 g/cm 3 . (a)
Question:
Magnesium oxide has the rock salt crystal structure and a density of 3.58 g/cm3.
(a) Determine the unit cell edge length.
(b) How does this result compare with the edge length as determined from the radii in Table 12.3, assuming that the Mg2+ and O2- ions just touch each other along the edges?
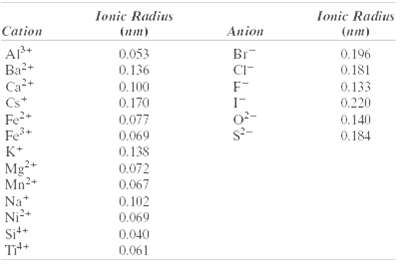
Transcribed Image Text:
Ionic Radius fonic Radius Cation Anion (nm) (nm) AP+ Ba+ Ca+ Br 0.053 0.196 0.181 0.136 CI- 0.133 0,220 F- 0.100 0.170 Cs* Fe+ Fe K+ 0.077 0.140 0.184 0.069 0.138 0.072 0.067 Mg+ Mn2+ Na* Ni² Sit+ 0.102 0.069 0.040 0.061
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (20 reviews)
a This part of the problem calls for us to determine the ...View the full answer

Answered By

Gaurav Soni
Teaching was always an area where I can pursue my passion. I used to teach my friends and junior during my school and college life. After completing my professional qualification (chartered accountancy) and before joining my job, I also joined an organization for teaching and guidance to my juniors. I had also written some articles during my internship which later got published. apart from that, I have also given some presentations on certain amendments/complex issues in various forms.
Linkedin profile link:
https://www.linkedin.com/in/gaurav-soni-38067110a
5.00+
7+ Reviews
13+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
Iron oxide (FeO) has the rock salt crystal structure and a density of 5.70 g/cm3. (a) Determine the unit cell edge length. (b) How does this result compare with the edge length as determined from the...
-
Magnesium (Mg) has an HCP crystal structure and a density of 1.74 g/cm3. (a) What is the volume of its unit cell in cubic centimeters? (b) If the c/a ratio is 1.624, compute the values of c and a.
-
Zirconium has an HCP crystal structure and a density of 6.51 g/cm 3 . (a) What is the volume of its unit cell in cubic meters? (b) If the c/a ratio is 1.593, compute the values of c and a.
-
True or False? Azure files can be accessed from anywhere in the world using a URL that points to the file. True False
-
Overall, studies have shown that stereotypes most often are accurate, with some notable exceptions. For example, political stereotypes are consistently inaccurate, while gender stereotypes tend to be...
-
Refer again to the financial statements for WestJet in Appendix II, looking at the consolidated statements of cash flows. Notice the same cash dividends paid during the year ended December 31, 2016...
-
A garden store prepares various grades of pine bark for mulch: nuggets (x1), mini-nuggets (x2), and chips (x3). The process requires pine bark, machine time, labor time, and storage space. The...
-
Goods Company is a major manufacturer of foodstuffs. The companys products are sold in grocery and convenience stores throughout the United States. Goods name is well known and respected because its...
-
Please answer all parts = Homework: Chapter M:8 Homework Question 2, EM8-12 (sim... Part 1 of 5 HW Score: 0%, 0 of 6 points : 0 O Points: 0 of 1 Save Rouse Builders builds 1,500-square-foot starter...
-
Mitchell Mineral Water, Inc., will pay a quarterly dividend per share of $.95 at the end of each of the next 12 quarters. Thereafter, the dividend will grow at a quarterly rate of 1 percent, forever....
-
Calculate the density of FeO, given that it has the rock salt crystal structure.
-
Compute the theoretical density of diamond given that the CC distance and bond angle are 0.154 nm and 109.5, respectively. How does this value compare with the measured density?
-
After teaching a group of students about RBC production, the instructor determines that the teaching was effective when the group states that the rate of RBC production is controlled by a. Iron. b....
-
Administrators at International University are curious how students' GPAs after their first year compare to their high school GPAs. They plan on taking an SRS of 80 of the 900 freshmen to look up...
-
( 8 x - x ^ 2 ) / ( x ^ 4 ) what is the derivate.
-
Solve for x . log 1 0 ( 4 x ) log 1 0 ( x 3 ) = 1
-
Let f ( x ) = ( 8 x - 4 x ^ 2 ) It is ^ x . Find the inflection points
-
f ( x ) = sin ( x ) / ( 2 * x ^ 2 + 4 ) , differentiate using quotient with respect to x
-
Elizabeth Perry, a student at SUNY, bought 6 bookcases for her dorm room. Each required unpacking of parts and assembly, which included some nailing and bolting. a) What is her learning rate? b)...
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
What property of diamond leads to the most engineering applications? Which types of applications would benefit from this property?
-
Is a brittle material a weak material?
-
What is the toughness of a material?
-
What is the difference between true stress and engineering stress? True strain and engineering strain?
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


