Question: Molecular weight data for some polymer are tabulated here. Compute (a) The number-average molecular weight, and (b) The weight-average molecular weight. (c) If it is
Molecular weight data for some polymer are tabulated here. Compute
(a) The number-average molecular weight, and
(b) The weight-average molecular weight.
(c) If it is known that this material's degree of polymerization is 710, which one of the polymers listed in Table 14.3 is this polymer? Why?
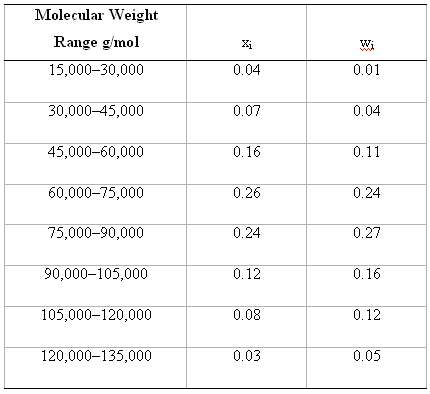
Molecular Weight Range g/mol Wi 15,000-30,000 0.04 0.01 0.07 30,000-45,000 0.04 0.11 45,000-60,000 0.16 60,000-75,000 0.26 0.24 75,000-90,000 0.27 0.24 0.16 0.12 90,000105,000 105,000-120,000 0.08 0.12 120,000135,000 0.03 0.05
Step by Step Solution
3.32 Rating (164 Votes )
There are 3 Steps involved in it
a From the tabulated data we are asked to compute the numberaverage molecular weight This is carried out be Molecular wt Range Mean M i x i x i M i 15... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (537).docx
120 KBs Word File


