Question: Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene at 40?C and 120 kPa absolute. In the process, dichlorobenzene (D) is generated
Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene at 40?C and 120 kPa absolute. In the process, dichlorobenzene (D) is generated as a co product: Liquid and gas streams leave the reactor. The liquid contains 49.2 wt% M, 29.6% D, and the remainder un-reacted B. The gas, which is sent to a treatment facility, contains 92% (v/v) HC1 and 8% un-reacted chlorine.
(a) What volume of gas leaves the reactor (m3/kg B fed)?
(b) The pipe through which the gas is to flow is sized so that the gas velocity is no greater than 10m/s. Derive an expression relating pipe diameter d (cm) to benzene feed rate mB0 (kg B/mm).
(c) In 1996, the demand for Monochlorobenzene was projected to decrease by 6%/year through the year 2000. What factors were contributing to the reduced demand when the projection was made?
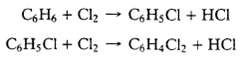
C6H6+ Cl C6HCl + Cl CHCl C6HCl + HCI + HCl
Step by Step Solution
3.25 Rating (186 Votes )
There are 3 Steps involved in it
Liquid composition 100 kg liquid 492 kg M 1 kmol 1126 kg 296 kg D 1 kmol 1470 k... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (203).pdf
180 KBs PDF File
13-E-C-E-C-P (203).docx
120 KBs Word File


